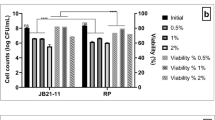
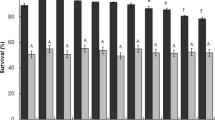
Abstract
Functional foods and nutraceuticals frequently contain viable probiotic strains that, at certain titers, are considered to be responsible of beneficial effects on health. Recently, it was observed that secreted metabolites might play a key role in this respect, especially in immunomodulation. Exopolysaccharides produced by probiotics, for example, are used in the food, pharmaceutical, and biomedical fields, due to their unique properties. Lactobacillus brevis CD2 demonstrated the ability to inhibit oral pathogens causing mucositis and periodontal inflammation and to reduce Helycobacter pylori infections. Due to the lack of literature, for this strain, on the development of fermentation processes that can increase the titer of viable cells and associated metabolites to industrially attractive levels, different batch and fed-batch strategies were investigated in the present study. In particular, aeration was shown to improve the growth rate and the yields of lactic acid and biomass in batch cultures. The use of an exponential feeding profile in fed-batch experiments allowed to produce 9.3 ± 0.45 × 109 CFU/mL in 42 h of growth, corresponding to a 20-fold increase of viable cells compared with that obtained in aerated batch processes; moreover, also increased titers of exopolysaccharides and lactic acid (260 and 150%, respectively) were observed. A purification process based on ultrafiltration, charcoal treatment, and solvent precipitation was applied to partially purify secreted metabolites and separate them into two molecular weight fractions (above and below 10 kDa). Both fractions inhibited growth of the known gut pathogen, Salmonella typhimurium, demonstrating that lactic acid plays a major role in pathogen growth inhibition, which is however further enhanced by the presence of Lact. brevis CD2 exopolysaccharides. Finally, the EPS produced from Lact. brevis CD2 was characterized by NMR for the first time up to date.





Similar content being viewed by others
References
Vesterlund S, Paltta J, Lauková A, Karp M, Ouwehand AC (2004) Rapid screening method for the detection of antimicrobial substances. J Microbiol Methods 57:23–31. https://doi.org/10.1016/j.mimet.2003.11.014
Mahdhi A, Leban N, Chakroun I, Chaouch MA, Hafsa J, Fdhila K, Mahdouani K, Majdoub H (2017) Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog 109:214–220. https://doi.org/10.1016/j.micpath.2017.05.046
Tapan Kumar S (2012) Microbial extracellular polymeric substances: production, isolation and applications. Iosr-phr 2:276–281. https://doi.org/10.9790/3013-0220276281
Schiraldi C, Valli V, Molinaro A, Cartenì M, De Rosa M (2006) Exopolysaccharides production in Lactobacillus bulgaricus and Lactobacillus casei exploiting microfiltration. J Ind Microbiol Biotechnol 33:384–390. https://doi.org/10.1007/s10295-005-0068-x
Caggianiello G, Kleerebezem M, Spano G (2016) Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol 100:3877–3886. https://doi.org/10.1007/s00253-016-7471-2
Khalil ES, Abd Manap MY, Mustafa S, Alhelli AM, Shokryazdan P (2018) Probiotic properties of exopolysaccharide-producing Lactobacillus strains isolated from Tempoyak. Molecules. 23:1–20. https://doi.org/10.3390/molecules23020398
Abid Y, Casillo A, Gharsallah H, Joulak I, Lanzetta R, Corsaro MM, Attia H, Azabou S (2019) Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int J Biol Macromol 108:719–728. https://doi.org/10.1016/j.ijbiomac.2017.10.155
Rahbar Saadat Y, Yari Khosroushahi A, Pourghassem Gargari B (2019) A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr Polym 217:79–89. https://doi.org/10.1016/j.carbpol.2019.04.025
Toh ZQ, Anzela A, Tang ML, Licciardi PV (2012) Probiotic therapy as a novel approach for allergic disease. Front Pharmacol 3:171–184. https://doi.org/10.3389/fphar.2012.00171
Sarowska J, Choroszy-Król I, Regulska-Ilow B, Frej-Mądrzak M, Jama-Kmiecik A (2013) The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med 22:759–766
Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G, Gaetani E, Sgambato A, Gasbarrini A (2013) Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int 435268:1–9. https://doi.org/10.1155/2013/435268
Fang F, Xu J, Li Q, Xia X, Du G (2018) Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol 18:221–229. https://doi.org/10.1186/s12866-018-1369-3
Hosono A, Lee JW, Ametani A, Natsume M, Hirayama M, Adachi T, Kaminogawa S (1997) Characterization of a water-soluble polysaccharide fraction with immunopotentiating activity from Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem 61:312–316. https://doi.org/10.1271/bbb.61.312
Li H, Qiu T, Huang G, Cao Y (2010) Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Factories 9:85–91. https://doi.org/10.1186/1475-2859-9-85
Karamese M, Aydin H, Sengul E, Gelen V, Sevim C, Ustek D, Karakus E (2016) The Immunostimulatory effect of lactic acid bacteria in a rat model. Iran J Immunol 13:220-228. IJIv13i3A7
Kitazawa H, Toba T, Itoh T, Kumano N, Adachi S, Yamaguchi T (1991) Antitumoral activity of slime-forming, encapsulated Lactococcus lactis subsp. cremoris isolated from scandinavian ropy sour milk, Viili. Anim Sci Technol 63:277–283. https://doi.org/10.3168/jds.S0022-0302(93)77483-4
Riaz Rajoka MS, Zhao H, Mehwish HM, Li N, Lu Y, Lian Z, Shao D, Jin M, Li Q, Zhao L, Shi J (2019) Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res Int 123:286–297. https://doi.org/10.1016/j.foodres.2019.05.002
Chuah LO, Foo HL, Loh TC, Mohammed Alitheen NB, Yeap SK, Abdul Mutalib NE, Abdul Rahim R, Yusoff K (2019) Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern Med 19:114–125. https://doi.org/10.1186/s12906-019-2528-2
Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, Namdar A (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol 234:17127–17143. https://doi.org/10.1002/jcp.28473
Nakajima H, Suzuki Y, Kaizu H (1992) Cholesterol lowering activity of ropy fermented milk. J Food Sci:1365–2621. https://doi.org/10.1111/j.1365-2621.1992.tb06848.x
Kitazawa H, Harata T, Uemura J, Saito T, Kaneko T, Itoh T (1998) Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int J Food Microbiol 40:169–175. 10.1016/s0168-1605(98)00030-0
Freitas F, Alves VD, Paism J, Costa N, Oliveira C, Mafrac L, Hilliou L, Oliveira R, Reis MAM (2009) Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol 100:859–865. https://doi.org/10.1016/j.biortech.2008.07.002
Shaofeng D, Tianwei T (2006) L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem 41:1451–1454. https://doi.org/10.1016/j.procbio.2006.01.014
Oleksy-Sobczak M, Klewicka E (2019) Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob Proteins:1–10. https://doi.org/10.1007/s12602-019-09581-2
Liu Q, Huang X, Yang D, Si T, Pan S, Yang F (2016) Yield improvement of exopolysaccharides by screening of the Lactobacillus acidophilus ATCC and optimization of the fermentation and extraction conditions. EXCLI J 15:119–133. https://doi.org/10.17179/excli2015-356
Abdelazez A, Abdelmotaal H, Evivie SE, Melak S, Jia FF, Khoso MH, Zhu ZT, Zhang LJ, Sami R, Meng XC (2018) Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on type I diabetes in vivo. Biomed Res Int 2018:1–20. https://doi.org/10.1155/2018/7356173
Suzuki S, Yakabe T, Suganuma H, Fukao M, Saito T, Yajima N (2013) Cell-bound exopolysaccharides of Lactobacillus brevis KB290: protective role and monosaccharide composition. Can J Microbiol 59:549–555. https://doi.org/10.1139/cjm-2013-0115
Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T (1996) Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J Am Coll Nutr 15:408–412. https://doi.org/10.1080/07315724.1996.10718617
Wu Q, Shah NP (2017) High γ-aminobutyric acid production from lactic acid bacteria: emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci Nutr 57:3661–3672. https://doi.org/10.1080/10408398.2016.1147418
Wu Q, Shap NP (2018) Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol 69:151–158. https://doi.org/10.1016/j.fm.2017.08.006
Fukao M, Oshima K, Morita H, Toh H, Suda W, Kim SW, Suzuki S, Yakabe T, Hattori M, Yajima N (2013) Genomic analysis by deep sequencing of the probiotic Lactobacillus brevis KB290 harboring nine plasmids reveals genomic stability. PLoS One 8(3):1–10. https://doi.org/10.1371/journal.pone.0060521
Fukao M, Zendo T, Inoue T, Nakayama J, Suzuki S, Fukaya T, Yajima N, Sonomoto K (2019) Plasmid-encoded glycosyltransferase operon is responsible for exopolysaccharide production, cell aggregation, and bile resistance in a probiotic strain, Lactobacillus brevis KB290. J Biosci Bioeng S1389-1723(19):30063–30065. https://doi.org/10.1016/j.jbiosc.2019.04.008
Murakami K, Habukawa C, Nobuta Y, Moriguchi N, Takemura T (2012) The effect of Lactobacillus brevis KB290 against irritable bowel syndrome: a placebo controlled double-blind crossover trial. Biopsychosoc Med 6:16–23. https://doi.org/10.1186/1751-0759-6-16
Fuke N, Aizawa K, Suganuma H, Takagi T, Naito Y (2017) Effect of combined consumption of Lactobacillus brevis KB290 and b-carotene on minor diarrhea-predominant irritable bowel syndrome-like symptoms in healthysubjects: a randomised, double-blind, placebo-controlled, parallel-group trial. Int J Food Sci Nutr 68:973–986. https://doi.org/10.1080/09637486.2017.1311843
Waki N, Matsumono M, Fukui Y, Suganuma H (2014) Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among school children. Lett Appl Microbiol 59:565–571. https://doi.org/10.1111/lam.12340
Sharma A, Tilak T, Bakhshi S, Raina V, Kumar L, Chaudhary SP, Sahoo RK, Gupta R, Thulkar S (2017) Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. SMO Open 1(6):e000138. https://doi.org/10.1136/esmoopen-2016-000138
Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A (2004) The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter 9:165–172. https://doi.org/10.1111/j.1083-4389.2004.00214.x
Riccia DN, Bizzini F, Perilli MG, Polimeni A, Trinchieri V, Amicosante G, Cifone MG (2007) Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis 13:376–378. https://doi.org/10.1111/j.1601-0825.2006.01291.x
Shrikant AS, Parag SS, Ishwar BB, Rekha S (2007) Fermentative production, downstream processing and applications. Food Technol Biotechnol 45:107–118
Vuotto C, Barbanti F, Mastrantonio P, Donelli G (2014) Lactobacillus brevis CD2 inhibits Prevotella melaninogenica biofilm. Oral Dis 20:668–674. https://doi.org/10.1111/odi.12186
Cimini D, Restaino OF, Catapano A, De Rosa M, Schiraldi C (2010) Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl Microbiol Biotechnol 85:1779–1787. https://doi.org/10.1007/s00253-009-2261-8
Scott TA, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25:1656–1661. https://doi.org/10.1021/ac60083a023
Restaino OF, Cimini D, Cassese E, Ventriglia R, Alfano A, Marrazzo A, D’ambrosio S, Barbuto Ferraiuolo S, De Rosa M, Schiraldi C (2019) Molecular weight determination of heparosan- and chondroitin-like capsular polysaccharides: figuring out differences between wild type and engineered Escherichia coli strains. Appl Microbiol Biotechnol 103:6771–6782. https://doi.org/10.1007/s00253-019-09969-8
Petry S, Furlan S, Waghorne E, Saulnier L, Cerning L, Maguin E (2003) Comparison of the thickening properties of four Lactobacillus delbrueckii subsp. bulgaricus strains and physicochemical characterization of their exopolysaccharides. FEMS Microbiol Lett 221:285–291. https://doi.org/10.1016/S0378-1097(03)00214-3
Casillo A, Ståhle J, Parrilli E, Sannino F, Mitchell DE, Pieretti G, Lanetta R, Parrilli M, Widmalm G, Tutino ML, Corsaro MM (2017) Structural characterization of an all-amino sugar-containing capsular polysaccharide from Colwellia psychrerythraea 34H. Antonie Leeuwenhoek 110:1377–1387. https://doi.org/10.1007/s10482-017-0834-6
Casillo A, Parrilli E, Sannino F, Mitchell DE, Gibson MI, Marino G, Lanzetta R, Parrilli M, Cosconati S, Novellino E, Randazzo A, Tutino ML, Corsaro MM (2017) Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: a strategy for cryoprotection. Carbohydr Polym 156:364–371. https://doi.org/10.1016/j.carbpol.2016.09.037
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Valgas C, de Souza SM, Smânia EFA, Smânia A Jr (2007) Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 38:369–380. https://doi.org/10.1590/S1517-83822007000200034
Lindgren SW, Dobrogosz WJ (1990) Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev 87:149–164. https://doi.org/10.1111/j.1574-6968.1990.tb04885.x
Wang Y, Tashiro Y, Sonomoto K (2015) Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J Biosci Bioeng 119:10–18. https://doi.org/10.1016/j.jbiosc.2014.06.003
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sc 7:222–229. https://doi.org/10.1016/j.jrras.2014.03.002
Aguirre-Ezkauriatza EJ, Aguilar-Yáñez JM, Ramírez-Medrano A, Alvarez MM (2010) Production of probiotic biomass (Lactobacillus casei) in goat milk whey: comparison of batch, continuous and fed-batch cultures. Bioresour Technol 101:2837–2844. https://doi.org/10.1016/j.biortech.2009.10.047
Juturu V, Wu JC (2018) Microbial production of bacteriocins: latest research development and applications. Biotechnol Adv 36:2187–2200. https://doi.org/10.1016/j.biotechadv.2018.10.007
Vivek N, Aswathi TV, Sven PR, Pandey A, Binod P (2017) Self-cycling fermentation for 1,3-propanediol production: comparative evaluation of metabolite flux in cell recycling, simple batch and continuous processes using Lactobacillus brevis N1E9.3.3 strain. J Biotechnol 259:110–119. https://doi.org/10.1016/j.jbiotec.2017.07.033
Hasegawa M, Yamane D, Funato K, Yoshida A, Sambongi Y (2018) Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. J Biosci Bioeng 125:316–319. https://doi.org/10.1016/j.jbiosc.2017.10.003
Xie C, Coda R, Chamlagain B, Varmanen P, Piironen V, Katina K (2019) Co-fermentation of Propionibacterium freudenreichii and Lactobacillus brevis in wheat bran for in situ production of vitamin B12. Front Microbiol 10:1541–1551. https://doi.org/10.3389/fmicb.2019.01541
Zhang Y, Vadlani PV (2015) Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J Biosci Bioeng 119:694–699. https://doi.org/10.1016/j.jbiosc.2014.10.027
Li CY, Lin HC, Lai CH, Lu JJ, Wu SF, Fang SH (2011) Immunomodulatory effects of Lactobacillus and Bifidobacterium on both murine and human mitogen-activated T cells. Int Arch Allergy Immunol 156:128–136. https://doi.org/10.1159/000322350
Neveling DP, Endo A, Dicks LMT (2012) Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Curr Microbiol 65:507–515. https://doi.org/10.1007/s00284-012-0186-4
Alfano A, Donnarumma G, Cimini D, Fusco A, Marzaioli I, De Rosa M, Schiraldi C (2015) Lactobacillus plantarum: microfiltration experiments for the production of probiotic biomass to be used in food and nutraceutical preparations. Biotechnol Prog 31:325–333. https://doi.org/10.1002/btpr.2037
Schiraldi C, Adduci V, Valli V, Maresca C, Giuliano M, Lamberti M, Carteni M, De Rosa M (2003) High cell density cultivation of probiotics and lactic acid production. Biotechnol Bioeng 2003:213–222. https://doi.org/10.1002/bit.10557
Schlothauer RC, Morgan AJ, Rademacher I, Christensen T, Martel I (2004) Use of lactobacillus to produce exopolysaccharides in food and pharmaceutical compositions WO2004013343 (A2)
Sun Kang T, Korber DR, Tanaka T (2013) Regulation of dual glycolytic pathways for fructose metabolism in heterofermentative Lactobacillus panis PM1. Appl Environ Microbiol 79:7818–7826. https://doi.org/10.1128/AEM.02377-13
Guo T, Zhang L, Xin Y, Xu Z, He H, Kong J (2017) Oxygen-inducible conversion of lactate to acetate in heterofermentative Lactobacillus brevis ATCC 367. Appl Environ Microbiol 17:83–104. https://doi.org/10.1128/AEM.01659-17
Brooijmans R, Smit B, Santos F, Riel JV, deVos WM, Hugenholtz J (2009) Heme and menaquinone induced electron transport in lactic acid bacteria. Microb Cell Factories 8:28–38. https://doi.org/10.1186/1475-2859-8-28
Callewaert R, De Vuyst L (2000) Bacteriocin production with Lactobacillus amylovorus DCE471 is improved and stabilized by fed-batch fermentation. Appl Environ Microbiol 66:606–613
Castro LP, Bernardez PF, Guerra NP, Guseva EV, Fick M (2007) Fed-batch pediocin production on whey using different feeding media. Enzym Microb Technol 41:397–406
Racine FM, Saha BC (2007) Production of mannitol by Lactobacillus intermedius NRRL B-3693 in fed-batch and continuous cell-recycle fermentations. Process Biochem 42:1609–1613
Polak-Bereckaa M, Waśko A, Skrzypekb H, Kreftc A (2013) Production of exopolysaccharides by a probiotic strain of Lactobacillus rhamnosus: biosynthesis and purification methods. Acta Aliment 42:220–228. https://doi.org/10.1556/AAlim.42.2013.2.9
Bajaj BI, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45:341–354
Freitas F, Alves VD, Reis AM (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29:388–398. https://doi.org/10.1016/j.tibtech.2011.03.008
Maekawa T, Hajishengallis G (2014) Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. Periodontal Res 49:785–791. https://doi.org/10.1111/jre.12164
Acknowledgments
The authors gratefully acknowledge Enrico Maria Cacciapuoti for fruitful discussions on fermentation and downstream processing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Bioteknet s.p.a. is a public body of which the L. Vanvitelli University is the majority shareholder.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alfano, A., Perillo, F., Fusco, A. et al. Lactobacillus brevis CD2: Fermentation Strategies and Extracellular Metabolites Characterization. Probiotics & Antimicro. Prot. 12, 1542–1554 (2020). https://doi.org/10.1007/s12602-020-09651-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09651-w