Abstract
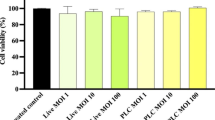
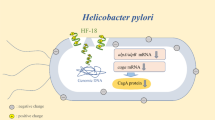
Helicobacter pylori is an infectious agent commonly associated with gastrointestinal diseases. The use of probiotics to treat this infection has been documented, however, their potential antimicrobial metabolites have not yet been investigated. In the present study, the effect of reuterin produced by Lactobacillus reuteri on H. pylori growth and virulence gene expression was evaluated. It was observed that reuterin caused significant (P < 0.05) H. pylori growth inhibition at concentrations from 0.08 to 20.48 mM, with minimal inhibitory concentrations (MICs) of 20.48 mM for H. pylori ATCC700824 and 10.24 mM for H. pylori ATCC43504. In a reuterin bacterial killing assay, it was observed that half of the MIC value for H. pylori (ATCC700824) significantly (P < 0.01) reduced colony numbers from 5.65 ± 0.35 to 3.78 ± 0.35 Log10 CFU/mL after 12 h of treatment and then increased them to 5.25 ± 0.23 Log10 CFU/mL at 24 h; at its MIC value (20.48 mM), reuterin abrogated (P < 0.01) H. pylori (ATCC700824) growth after 20 h of culture. In addition, reuterin significantly (P < 0.01) reduced H. pylori (ATCC 43504) colony numbers from 5.65 ± 0.35 to 4.1 ± 0.12 Log10 CFU/mL from 12 to 24 h of treatment and abrogated its growth at its MIC value (10.24 mM), after 20 h of treatment. Reuterin did not alter normal human gastric Hs738.St/Int cell viability at the concentrations tested for H. pylori strains. Furthermore, 10 μM reuterin was shown to significantly (P < 0.01) reduce mRNA relative expression levels of H. pylori virulence genes vacA and flaA at 3 h post-treatment, whose effect was higher at 6 h post-treatment, as measured by RT-qPCR. The observed direct antimicrobial effect and the downregulation of expression of virulence genes on H. pylori by reuterin may contribute to the understanding of the mechanisms of action of probiotics against H. pylori.




Similar content being viewed by others
References
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1(8390):1311–1315. https://doi.org/10.1016/S0140-6736(84)91816-6
Kusters JG, Van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19(3):449–490. https://doi.org/10.1128/CMR.00054-05
International Agency for Research on Cancer (1994) Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 61:1–241
Amieva MR, El-Omar EM (2008) Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134(1):306–323. https://doi.org/10.1053/j.gastro.2007.11.009
Wroblewski LE, Peek RM Jr, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23(4):713–739. https://doi.org/10.1128/CMR.00011-10
Chiarini A, Cala C, Bonura C, Gullo A, Giuliana G, Peralta S, D'Arpa F, Giammanco A (2009) Prevalence of virulence-associated genotypes of Helicobacter pylori and correlation with severity of gastric pathology in patients from western Sicily, Italy. Eur J Clin Microbiol Infect Dis 28(5):437–446. https://doi.org/10.1007/s10096-008-0644-x
Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, European Helicobacter Study G (2012) Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 61(5):646–664. https://doi.org/10.1136/gutjnl-2012-302084
Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA (2016) Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43(4):514–533. https://doi.org/10.1111/apt.13497
Casas IA, Dobrogosz WJ (2000) Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 12(4):247–285. https://doi.org/10.3402/mehd.v12i4.8196
Francavilla R, Lionetti E, Castellaneta SP, Magista AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E, Miniello VL (2008) Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter 13(2):127–134. https://doi.org/10.1111/j.1523-5378.2008.00593.x
Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A, Cavallo L, Francavilla A, Versalovic J (2014) Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol 48(5):407–413. https://doi.org/10.1097/MCG.0000000000000007
Dore MP, Soro S, Rocchi C, Loria MF, Bibbo S, Pes GM (2016) Inclusion of Lactobacillus reuteri in the treatment of Helicobacter pylori in Sardinian patients. Medicine 95(15):1–3. https://doi.org/10.1097/MD.0000000000003411
Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition (2010) Probiotics and prebiotics in pediatrics. Pediatrics 126(6):1217–1231. https://doi.org/10.1542/peds.2010-2548
Vollenweider S, Grassi G, Konig I, Puhan Z (2003) Purification and structural characterization of 3-hydroxypropionaldehyde and its derivatives. J Agric Food Chem 51(11):3287–3293. https://doi.org/10.1021/jf021086d
Talarico TL, Casas IA, Chung TC, Dobrogosz WJ (1988) Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother 32(12):1854–1858. https://doi.org/10.1128/AAC.32.12.1854
Talarico TL, Dobrogosz WJ (1989) Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob Agents Chemother 33(5):674–679. https://doi.org/10.1128/AAC.33.5.674
Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G (2007) Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol 7(1):101. https://doi.org/10.1186/1471-2180-7-101
Vollenweider S, Lacroix C (2004) 3-Hydroxypropionaldehyde: applications and perspectives of biotechnological production. Appl Microbiol Biotechnol 64(1):16–27. https://doi.org/10.1007/s00253-003-1497-y
Ganzle MG (2004) Reutericyclin: biological activity, mode of action, and potential applications. Appl Microbiol Biotechnol 64(3):326–332. https://doi.org/10.1007/s00253-003-1536-8
Delgado S, Leite AM, Ruas-Madiedo P, Mayo B (2014) Probiotic and technological properties of Lactobacillus spp. strains from the human stomach in the search for potential candidates against gastric microbial dysbiosis. Front Microbiol 5:766. https://doi.org/10.3389/fmicb.2014.00766
Li J, Wang W, Xu SX, Magarvey NA, McCormick JK (2011) Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc Natl Acad Sci U S A 108(8):3360–3365. https://doi.org/10.1073/pnas.1017431108
Salehi R, Savabi O, Kazemi M, Kamali S, Salehi AR, Eslami G, Tahmourespour A (2014) Effects of Lactobacillus reuteri-derived biosurfactant on the gene expression profile of essential adhesion genes (gtfB, gtfC and ftf) of Streptococcus mutans. Adv Biomed Res 3:169. https://doi.org/10.4103/2277-9175.139134
Ryan KA, O’Hara AM, van Pijkeren JP, Douillard FP, O’Toole PW (2009) Lactobacillus salivarius modulates cytokine induction and virulence factor gene expression in Helicobacter pylori. J Med Microbiol 58(Pt 8):996–1005. https://doi.org/10.1099/jmm.0.009407-0
Baca-Castanon ML, De la Garza-Ramos MA, Alcazar-Pizana AG, Grondin Y, Coronado-Mendoza A, Sanchez-Najera RI, Cardenas-Estrada E, Medina-De la Garza CE, Escamilla-Garcia E (2015) Antimicrobial effect of Lactobacillus reuteri on cariogenic bacteria Streptococcus gordonii, Streptococcus mutans, and periodontal diseases Actinomyces naeslundii and Tannerella forsythia. Probiotics Antimicrob Proteins 7(1):1–8. https://doi.org/10.1007/s12602-014-9178-y
Circle SJ, Stone L, Boruff CS (1945) Acrolein determination by means of tryptophane. A colorimetric micromethod. Ind Eng Chem Anal Ed 17(4):259–262. https://doi.org/10.1021/i560140a021
Rütti DP, Lacroix C, Jeremiç T, Mathis M, Díe A, Vollenweider S (2011) Development of a reversible binding process for in situ removal of 3-hydroxypropionaldehyde during biotechnological conversion of glycerol. Biochem Eng J 55(3):176–184. https://doi.org/10.1016/j.bej.2011.04.005
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicron Chemother 48(Suppl 1):5–16. https://doi.org/10.1093/jac/dkf083
CLSI (2015) Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25. Committee for Clinical Laboratory Standards, Wayne, PA, 35(3):1–236
Koeth LM (2016) Tests to assess bactericidal activity. Clinical Microbiology Procedures Handbook, vol 2. ASM press, Washington, DC, pp 950–978 ISBN: 9781555818807
Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swenson JM (1999) Methods for determining bactericidal activity of antimicrobial agents: approved guideline. NCCLS document M26-A 19(18):1–31 ISBN: 1-56238-384-1
Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD (2007) Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacolo Ther 25(2):155–168. https://doi.org/10.1111/j.1365-2036.2006.03179.x
Arqués JL, Fernández J, Gaya P, Nuñez M, Rodrı́guez E, Medina M (2004) Antimicrobial activity of reuterin in combination with nisin against food-borne pathogens. Int J Food Microbiol 95(2):225–229. https://doi.org/10.1016/j.ijfoodmicro.2004.03.009
El-Ziney MG, van den Tempel T, Debevere J, Jakobsen M (1999) Application of reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation. J Food Protect 62(3):257–261. https://doi.org/10.4315/0362-028X-62.3.257
Chung TC, Axelsson L, Lindgren SE, Dobrogosz WJ (1989) In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Ecol Health Dis 2(2):137–144. https://doi.org/10.3109/08910608909140211
Liang HF, Chen CN, Chang Y, Sung HW (2003) Natural antimicrobial agent (reuterin) produced by Lactobacillus reuteri for sanitization of biological tissues inoculated with Pseudomonas aeruginosa. Biotechnol Bioeng 84(2):233–239. https://doi.org/10.1002/bit.10764
El-Ziney MG, Debevere JM (1998) The effect of reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in milk and cottage cheese. J Food Protect 61(10):1275–1280. https://doi.org/10.4315/0362-028X-61.10.1275
Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J (2008) Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14(3):166–171. https://doi.org/10.1016/j.anaerobe.2008.02.001
Laughton JM, Devillard E, Heinrichs DE, Reid G, McCormick JK (2006) Inhibition of expression of a staphylococcal superantigen-like protein by a soluble factor from Lactobacillus reuteri. Microbiology 152(Pt 4):1155–1167. https://doi.org/10.1099/mic.0.28654-0
Eaton KA, Suerbaum S, Josenhans C, Krakowka S (1996) Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun 64(7):2445–2448
Rader BA, Campagna SR, Semmelhack MF, Bassler BL, Guillemin K (2007) The quorum-sensing molecule autoinducer 2 regulates motility and flagellar morphogenesis in Helicobacter pylori. J Bacteriol 189(17):6109–6117. https://doi.org/10.1128/JB.00246-07
Dobrogosz WJ, Lindgren SE (1988) Antibiotic Reuterin. Patent EP0357673A1, PCT/US1988/001423. https://patentscope.wipo.int/search/docservicepdf_pct/id00000000827490/PAMPH/WO1988008452.pdf
Schauenstein E, Esterbauer H, Zollner H (1977) Saturated aldehydes. Aldehydes in biological systems: their natural occurrence and biological activities, vol 5. Pion, London, pp 9–24 ISBN: 0850860598
Morita H, Toh H, Fukuda S, Horikawa H, Oshima K, Suzuki T, Murakami M, Hisamatsu S, Kato Y, Takizawa T, Fukuoka H, Yoshimura T, Itoh K, O’Sullivan DJ, McKay LL, Ohno H, Kikuchi J, Masaoka T, Hattori M (2008) Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res 15(3):151–161. https://doi.org/10.1093/dnares/dsn009
Acknowledgements
We thank Laboratorio de Inmunología y Virología of Facultad de Ciencias Biológicas at Universidad Autónoma de Nuevo León for supporting the development of this study.
Funding
This study was partly supported by grants from Programa de Apoyo a la Investigación Científica y Tecnológica from Universidad Autónoma de Nuevo León (PAICYT-UANL) to RGF and by Consejo Nacional de Ciencia y Tecnología (CONACYT-México) to VUB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Urrutia-Baca, V.H., Escamilla-García, E., de la Garza-Ramos, M.A. et al. In Vitro Antimicrobial Activity and Downregulation of Virulence Gene Expression on Helicobacter pylori by Reuterin. Probiotics & Antimicro. Prot. 10, 168–175 (2018). https://doi.org/10.1007/s12602-017-9342-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9342-2