Abstract
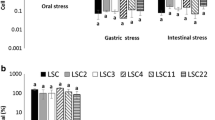
This study was conducted to evaluate the probiotic properties of Lactobacillus reuteri isolated from human infant feces (less than 3 months). Out of thirty-two representative L. reuteri strains isolated from the infant human feces, nine isolates (i.e. LR5, LR6, LR9, LR11, LR19, LR20, LR25, LR26 and LR34) showed survival in acid, bile and simulated stomach–duodenum passage conditions, indicating their high tolerance to gastric juice, duodenal juice and bile environments. The nine isolates did not show strong hydrophobic properties because the percentages of adhesion to the apolar solvent, n-hexadecane, did not exceed 40%, showing that their surfaces were rather hydrophilic. Functionality of these nine probiotic isolates was supported by their antagonistic activity and their ability to deconjugate bile salts. The safety of the nine indigenous L. reuteri isolates was supported by the absence of transferable antibiotic resistance determinants, DNase activity, gelatinase activity and hemolysis. The results obtained so far suggest that the nine strains are resistant to low pH, bile salts and duodenum juice, so they could survive when passing through the upper part of the gastrointestinal tract and fulfill their potential probiotic action in the host organism. According to these results, the L. reuteri strains isolated from human infant feces possess interesting probiotic properties that make them potentially good candidates for probiotics.


Similar content being viewed by others
References
Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, Oldaeus G (2007) Probiotics in prevention of IgE-associated eczema: a double blind randomized placebo-controlled trial. J Allergy Clin Immunol 119:1174–1180
Bezkorovainy A (2001) Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr 73:399S–405S
Biswas SR, Ray P, Johnson MC, Ray B (1991) Influence of growth conditions on the production of a bacteriocin AcH by Pediococcus acidilactici H. Appl Environ Microbiol 5:1265–1267
Bogovic Matijasic B, Rogelj I (2000) Lactobacillus K7—a new candidate for a probiotic strain. Food Technol Biotechnol 38(2):113–119
Carey CM, Kostrzynska M, Ojha S, Thompson S (2008) The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J Microbiol Methods 73:125–132
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Development and application of an in vivo methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84:759–768
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Ingredient selection criteria for probiotic microorganisms in functional dairy foods. Int J Dairy Technol 51(4):123–136
Charteris WP, Kelly PM, Morelli L, Collins JK (2001) Gradient diffusion antibiotic susceptibility testing of potentially probiotic lactobacilli. J Food Prot 64:2007–2014
Chou L, Weimer B (1999) Isolation and characterization of acid and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci 82:23–31
Christensen HR, Frokiaer H, Pestka JJ (2002) Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 168:171–178
Clark PA, Cotton LN, Martin JH (1997) Selection of Bifidobacteria for use as delivery adjuncts in cultured dairy foods. II. Tolerance to stimulated pH of human stomachs. J Cultured Dairy Prod 28(4):11–14
Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G (2007) Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol 7:101
Clinical and Laboratory Standards Institute (CLSI) (2009) In: Jeffrey L, Thomas R, Michael Apley, Donald J, Steven D et al. (eds) Performance standards for antimicrobial disk susceptibility tests, vol 28(8). pp 13–23
Conway PL, Gorbach SL, Goldin BR (1987) Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci 70:1–12
Corzo G, Gilliland SE (1999) Measurements of bile salt hydrolase activity from Lactobacillus acidophilus based on disappearance of conjugated bile salts. J Dairy Sci 82:466–471
Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82:1–11
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of Lactobacilli. J Appl Microbiol 23(l):130–135
De Smet I, Van Hoorde L, Vande Woestyne M, Cristianes H, Verstraete W (1995) Significance of bile salt hydrolytic activities of Lactobacilli. J Appl Bacteriol 79:292–301
Dubernet S, Desmasures N, Guéguen M (2002) A PCR-based method for identification of Lactobacilli at the genus level. FEMS Microbiol Lett 214:271–275
Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C, Kiely B, Quigley EM, O’Sullivan GC, Shanahan F, Collins JK (1999) Probiotics: from myth to reality. Demonstration of functionality on animal models of disease and in human clinical trials. Anton Leeuwen 76:279–292
Dunne C, O’Mahony L, Murphy L, Thornton G, Morrisey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, Kiely B, O’Sullivan GC, Shanahan F, Collins JK (2001) In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 73:386S–392S
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina
FAO/WHO (2002) Joint FAO/WHO working group meeting report on drafting guidelines for the evaluation of probiotics in food, London Ontario, Canada, pp 1–28
Fernandez MF, Boris S, Barbes C (2003) Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
Fleming HP, Etchells JL, Costilow RN (1975) Microbial inhibition by an isolate of Pediococcus from cucumber brines. Appl Microbiol 30:1040–1042
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Fuller R (1992) History and development of probiotics. In: Fuller R (ed) Probiotics. Chapman and Hall, Cambridge, pp 1–8
Garg KB, Ganguli I, Das R, Talwar GP (2009) Spectrum of Lactobacillus species present in healthy vagina of Indian women. Indian J Med Res 29:652–657
Gilliland SE, Staley TE, Bush LJ (1984) Importance of bile tolerance of Lactobacillus acidophilus used as dietary adjuct. J Dairy Sci 67:3045–3055
Gilliland SE, Walker DK (1990) Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J Dairy Sci 73:905–911
Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gaulthierri L, Salminer S (1992) Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37:121–128
Gupta H, Malik RK (2007) Incidence of virulence in bacteriocin producing enterococcal isolates. Lait 87:587–601
Haung Y, Adams MC (2004) In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol 91:253–260
Hoffman AF, Molino G, Milanese M, Belforte G (1983) Description and stimulation of a physiological pharmokinetic model for the metabolism and enterohepatic circulation of bile acids in man. J Clin Invest 71:1003–1025
Hofmann A (1991) Enterohepatic circulation of bile acids. In: Schultz SG, Forte JG, Rauner BB (eds) Handbook of physiology. Section 6: the gastrointestinal system, salivary, gastric, pancreatic, and hepatobiliary secretion, vol 3, pp 567–580
Imase K, Tanaka A, Tokunaga K, Sugano H, Ishida H, Takahashi S (2007) Lactobacillus reuteri tablets suppress Helicobacter pylori infection—a double-blind, randomized, placebo-controlled cross-over clinical study. J Jpn Assoc Infect Dis 81:387–393
Jacobsen CN, Nielsen R, Hayford AE, Moller PL, Michaelsen KF, Aerregaard AP, Sandstrom B, Tvede M, Jakobsen M (1999) Screening of probiotic activities of forty seven strains of Lactobacillus species by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65(11):49–56
Jin LZ, Ho YW, Abdullah N, Jalaludin S (1998) Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett Appl Microbiol 27:183–185
Katla AK, Kruse H, Johnsen G, Herikstadt H (2001) Antimicrobial susceptibility of starter culture bacteria in Norwegian dairy products. Int J Food Microbiol 67:147–152
Kiely LJ, Olson NF (2000) The physicochemical surface characteristics of Lactobacillus casei. Food Microbiol 17:277–291
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–85
Klaenhammer TR, Kleeman EG (1981) Growth characteristics, bile sensitivity and freeze damage in colonial variants of Lactobacillus acidophilus. Appl Environ Microbiol 41(6):1461–1467
Kociubinsky G, Perez P, De Antoni G (1999) Screening of bile resistance and bile precipitation in lactic acid bacteria and bifidobacteria. J Food Prot 62:905–912
Lankaputhra WEV, Shah NP (1995) Survival of Lactobacillus acidophilus and Bifidobacterium spp. in presence of acid and bile salts. J Cultured Dairy Prod 30:7–8
Lindgren SE, Dobrogosz WJ (1990) Antagonistic activities of lactic acid bacteria in food and feed fermentation. FEMS Microbiol Rev 87:149–164
Liu Y, Fatheree NY, Mangalat N, Rhoads JM (2010) Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastroint Liver Physiol 299(5):G1087–G1096
Marteau P, Gerhardt MF, Myara A, Bouvier E, Trivin F, Rambaud JC (1995) Metabolism of bile salts by alimentary bacteria during transit in the human small intestine. Microbial Ecol Health Dis 8:151–157
Mathara JM, Schillinger U, Guigas C, Franz C, Kutima PM, Mbugua SK, Shin HK, Holzapfel WH (2008) Functional characteristics of Lactobacillus spp. from traditional Maasai fermented milk products in Kenya. Int J Food Microbiol 126:57–64
Mishra V, Prasad DN (2005) Application of in vitro methods for selection of Lactobacillus casei. Int J Food Microbiol 103(1):109–115
Morata De Ambrosini V, Gonzalez S, De Ruiz Holgando AP, Oliver G (1998) Study of morphology of cells of some strains of lactic acid bacteria and related species. J Food Prot 61:557–562
Morelli L, Cesena C, De Haen C, Gozzini L (1998) Taxonomic Lactobacillus composition of feces from human newborns during the first few days. Microbial Ecol 35:205–212
Moser SA, Savage D (2001) Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Appl Environ Microbiol 67(8):3476–3480
Pospiech A, Neumann B (1995) A versatile quick preparation of genomic DNA from gram positive bacteria. Trends Genetics 11:217–218
Reuter G (2001) The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Iss Intest Microbiol 2:43–53
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Schillinger U, Guigas C, Holzapfel WH (2005) In vitro adherence and other properties of lactobacilli used in probiotic yoghurt like products. Int Dairy J 15:1289–1297
Smits HH, Engering A, Van der Kleij D, De Jong EC, Schipper K, van Capel TMM, Zaat BAJ, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg L (2005) Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cells specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol 115:1260–1267
Song YL, Kato N, Liu CX, Kato H, Watanabe K (1999) Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol 37:3062–3064
Song YL, Kato N, Liu CX, Matsumiya Y, Kato H, Watanabe K (2000) Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett 187:167–173
Suja S (2003) Development of PCR techniques for the identification of dairy lactobacilli. Dissertation, N.D.R.I. Karnal, India
Tannock GW, Dashkevitz MP, Feighner SD (1989) Lactobacilli and bile salt hydrolase in the murine intestinal tract. Appl Environ Microbiol 55:1848–1851
Tannock GW, Munro K, Harmsen HJM, Welling GW, Smart J, Gopal PK (2000) Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol 66:2578–2588
Taranto MP, de Ruiz Holgado AP, Valdez GF (1995) Bile salt hydrolase activity of Enterococcus faecium strains. Microbiol Aliments Nut 13:345–375
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K (2004) Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70:1176–1181
Vinderola CG, Reinheimer JA (2003) Lactic acid starters and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int 36:895–904
Zarate G, Perez Chaia A, Gonzalez S, Oliver G (2000) Viability and β-galactosidase activity of dairy propionibacteria subjected to digestion by artificial gastric and intestinal fluids. J Food Prot 63:1214–1221
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, T.P., Kaur, G., Malik, R.K. et al. Characterization of Intestinal Lactobacillus reuteri Strains as Potential Probiotics. Probiotics & Antimicro. Prot. 4, 47–58 (2012). https://doi.org/10.1007/s12602-012-9090-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-012-9090-2