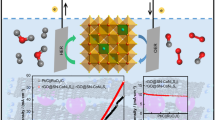
Abstract
Developing efficient oxygen evolution reaction (OER) electrocatalysts such as transition metal sulfides (TMSs) is of great importance to advance renewable hydrogen fuel toward further practical applications. Herein, NiCoS2 nanoparticles well decorated on double-sided N-doped reduced graphene oxide sheets (NiCoS2/rGO) are prepared from an Al-containing ternary NiCoAl-layered double hydroxide precursor (NiCoAl-LDH) grown on GO support as an OER electrocatalyst. The Al-confinement-assisted sulfurization, followed by selective acid treatment, endows the resulting NiCoS2/rGO composite with the advantages: well-dispersed NiCoS2 nanoparticles, dual-sided rGO support, as well as a large specific surface area of 119.4 m2·g–1 and meso-/macroporous size distribution. The NiCoS2/rGO electrocatalyst exhibits an overpotential of 273 mV at 10 mA·cm–2 and a good stability of 24 h, which outperform those of the counterparts of NiS2/rGO and CoS2/rGO. The results of electrochemical active surface area and electrochemical impedance spectra experimentally provide convincing rationales of the information of active sites and good conductivity, both underpin the enhanced electrocatalytic performances.
Graphical abstract

摘要 (Chinese abstract)
开发高效析氧反应 (OER) 电催化剂 (如过渡金属硫化物) 对于推动可再生氢能源的进一步实际应用具有重要意义。本研究以氧化石墨烯 (GO) 载体上负载含铝三元镍钴铝层状双氢氧化物 (NiCoAl-LDH) 为前驱体, 制备得到负载于氮掺杂还原氧化石墨烯载体两侧的NiCoS2纳米颗粒 (NiCoS2/rGO) OER催化剂。借助非活性铝的限域作用及选择性酸处理后, 该复合材料具有以下优点: 分散性良好的NiCoS2纳米颗粒、双面负载的rGO载体、较大比表面积 (119.4 m2·g–1) 和介孔/大孔尺寸分布。该复合OER电催化剂在10 mA·cm–2时的过电位为273 mV, 并且24 h后保持良好循环稳定性, 表现出明显优于单组分NiS2/rGO和CoS2/rGO的OER性能。OER性能提高的原因在于材料具有较大的电化学活性面积与较低的电荷转移电阻.






Similar content being viewed by others
References
Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff I, Norskov JK, Jaramillo TF. Combining theory and experiment in electrocatalysis: insights into materials design. Science. 2017;355(6321):4998.
Song J, Wei C, Huang ZF, Liu C, Zeng L, Wang X, Xu ZJ. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem Soc Rev. 2020;49(7):2196.
Wang Q, Liu D, He X. Metal-organic framework-derived Fe-N-C nanohybrids as highly-efficient oxygen reduction catalysts. Acta Phys Chim Sin. 2019;35(7):740.
Qiao MF, Wang Y, Li L, Hu GZ, Zou GA, Mamat X, Dong YM, Hu X. Self-templated nitrogen-doped mesoporous carbon decorated with double transition-metal active sites for enhanced oxygen electrode catalysis. Rare Met. 2019;39(7):824.
Chen X, Wang H, Meng R, Xia B, Ma Z. Cadmium hydroxide: a missing non-noble metal hydroxide electrocatalyst for the oxygen evolution reaction. ACS Appl Energy Mater. 2020;3(2):1305.
Li H, Xu SM, Yan H, Yang L, Xu S. Cobalt phosphide composite encapsulated within N, P-doped carbon nanotubes for synergistic oxygen evolution. Small. 2018;14(19):1800367.
Pothu R, Bolagam R, Wang QH, Ni W, Cai JF, Peng XX, Feng YZ, Ma JM. Nickel sulfide-based energy storage materials for high-performance electrochemical capacitors. Rare Met. 2021;40(2):353.
Manjunatha C, Srinivasa N, Chaitra SK, Sudeep M, Chandra Kumar R, Ashoka S. Controlled synthesis of nickel sulfide polymorphs: studies on the effect of morphology and crystal structure on OER performance. Mater Today Energy. 2020;16:100414.
Li K, Qian Y, Zhang H, Zhang L, Chai Q, Wang Q, Du J, Han Y, Wang W, Kang DJ. Highly efficient oxygen evolution electrocatalysts based on nanosheet-shaped CuS in situ grown on carbon cloth. Ceram Int. 2019;45(8):10664.
Zhang D, Mou H, Lu F, Song C, Wang D. A novel strategy for 2D/2D NiS/graphene heterostructures as efficient bifunctional electrocatalysts for overall water splitting. Appl Catal B. 2019;254:471.
Wj D, Pan Y, Wang N, Sk Wu, XZ Li, Zhu YA, Lu T. Nanocrystalline NiS particles synthesized by mechanical alloying as a promising oxygen evolution electrocatalyst. Mater Lett. 2018;218:115.
Feng X, Jiao Q, Li Q, Shi Q, Dai Z, Zhao Y, Li H, Feng C, Zhou W, Feng T. NiCo2S4 spheres grown on N, S co-doped rGO with high sulfur vacancies as superior oxygen bifunctional electrocatalysts. Electrochim Acta. 2020;331:135356.
Xin Y, Kan X, Gan LY, Zhang Z. Heterogeneous bimetallic phosphide/sulfide nanocomposite for efficient solar-energy-driven overall water splitting. ACS Nano. 2017;11(10):10303.
Tang Y, Yang H, Sun J, Xia M, Guo W, Yu L, Yan J, Zheng J, Chang L, Gao F. Phase-pure pentlandite Ni4.3Co4.7S8 binary sulfide as an efficient bifunctional electrocatalyst for oxygen evolution and hydrogen evolution. Nanoscale. 2018;10(22):10459.
Wang JY, Ouyang T, Li N, Ma T, Liu ZQ. S, N co-doped carbon nanotube-encapsulated core-shelled CoS2@Co nanoparticles: efficient and stable bifunctional catalysts for overall water splitting. Sci Bull. 2018;63(17):1130.
Ren JT, Yuan ZY. Bifunctional electrocatalysts of cobalt sulfide nanocrystals in situ decorated on N, S-codoped porous carbon sheets for highly efficient oxygen electrochemistry. ACS Sustain Chem Eng. 2019;7(11):10121.
Ma M, Yang G, Wang H, Lu Y, Zhang B, Cao X, Peng D, Du X, Liu Y, Huang Y. Ordered distributed nickel sulfide nanoparticles across graphite nanosheets for efficient oxygen evolution reaction electrocatalyst. Int J Hydr Energy. 2019;44(3):1544.
Jiang J, Lu S, Wang WK, Huang GX, Huang BC, Zhang F, Zhang YJ, Yu HQ. Ultrahigh electrocatalytic oxygen evolution by iron-nickel sulfide nanosheets/reduced graphene oxide nanohybrids with an optimized autoxidation process. Nano Energy. 2018;43:300.
Wang ZY, Jiang SD, Duan CQ, Wang D, Luo SH, Liu YG. In situ synthesis of Co3O4 nanoparticles confined in 3D nitrogen-doped porous carbon as an efficient bifunctional oxygen electrocatalyst. Rare Met. 2020;39(12):1383.
Wang N, Li L, Zhao D, Kang X, Tang Z, Chen S. Graphene composites with cobalt sulfide: efficient trifunctional electrocatalysts for oxygen reversible catalysis and hydrogen production in the same electrolyte. Small. 2017;13(33):1701025.
Li J, Liu G, Fu J, Gp J, Luo D, Hassan FM, Zhang J, Deng YP, Xu P, Ricardez-Sandoval L, Zw C. Surface decorated cobalt sulfide as efficient catalyst for oxygen evolution reaction and its intrinsic activity. J Catal. 2018;367:43.
Zhang W, Cui L, Liu J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J Alloy Compd. 2020;821:153542.
Huang ZF, Wang J, Yc P, Jung CY, Fisher A, Wang X. Design of efficient bifunctional oxygen reduction/evolution electrocatalyst: recent advances and perspectives. Adv Energy Mater. 2017;7(23):1700544.
Chauhan M, Reddy KP, Gopinath CS, Deka S. Copper cobalt sulfide nanosheets realizing a promising electrocatalytic oxygen evolution reaction. ACS Catal. 2017;7(9):5871.
Yao N, Li P, Zhou Z, Meng R, Cheng G, Luo W. Nitrogen engineering on 3D dandelion-flower-like CoS2 for high-performance overall water splitting. Small. 2019;15(31):1901993.
Xiang W, Tian Q, Zhong C, Deng Y, Han X, Hu W. A Solution-based method for synthesizing pyrite-type ferrous metal sulfide microspheres with efficient OER activity. Chem Asian J. 2020;15(14):2231.
Zhang J, Wang T, Xue D, Guan C, Xi P, Gao D, Huang W. Energy-level engineered hollow N-doped NiS1.03 for Zn–Air batteries. Energy Storage Mater. 2020;25:202.
Xu Y, Sumboja A, Zong Y, Darr JA. Bifunctionally active nanosized spinel cobalt nickel sulfides for sustainable secondary zinc–air batteries: examining the effects of compositional tuning on OER and ORR activity. Catal Sci Technol. 2020;10(7):2173.
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Wei Lu, Tour JM. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8):4806.
Zhou L, Shao M, Zhang C, Zhao J, He S, Rao D, Wei M, Evans DG, Duan X. Hierarchical CoNi-sulfide nanosheet arrays derived from layered double hydroxides toward efficient hydrazine electrooxidation. Adv Mater. 2017;29(6):1604080.
Zhang X, Zhao Y, Zhao Y, Shi R, Waterhouse GIN, Zhang T. A simple synthetic strategy toward defect-rich porous monolayer NiFe-layered double hydroxide nanosheets for efficient electrocatalytic water oxidation. Adv Energy Mater. 2019;9(24):1990081.
Jia X, Zhang X, Zhao J, Zhao Y, Zhao Y, Waterhouse GIN, Shi R, Wu LZ, Tung CH, Zhang T. Ultrafine monolayer co-containing layered double hydroxide nanosheets for water oxidation. J Energy Chem. 2019;34:57.
Rebekah A, Ashok Kumar E, Viswanathan C, Ponpandian N. Effect of cation substitution in MnCo2O4 spinel anchored over rGO for enhancing the electrocatalytic activity towards oxygen evolution reaction (OER). Int J Hydr Energy. 2020;45(11):6391.
Qin PL, Zeng K, Lan ZQ, Huang XT, Liu HZ, Guo J. Enhanced dydrogen storage properties of Mg–Al Alloy catalyzed with reduced graphene oxide supported with LaClO. Chin J Rare Metals. 2020;44(5):499.
Fan G, Li F, Evans DG, Duan X. Catalytic applications of layered double hydroxides: recent advances and perspectives. Chem Soc Rev. 2014;43(20):7040.
Liang Z, Huo R, Yin YX, Zhang F, Xu S, Guo YG. Carbon-supported Ni@NiO/Al2O3 integrated nanocomposite derived from layered double hydroxide precursor as cycling-stable anode materials for lithium-ion batteries. Electrochim Acta. 2013;108:429.
Jiang S, Ithisuphalap K, Zeng X, Wu G, Yang H. 3D porous cellular NiCoO2/graphene network as a durable bifunctional electrocatalyst for oxygen evolution and reduction reactions. J Power Sour. 2018;399:66.
Liu Y, Su D, Sang Z, Su X, Chen H, Yan X. High-performance layered NiCo2S4@rGO/rGO film electrode for flexible electrochemical energy storage. Electrochim Acta. 2019;328:135088.
Cui Y, Zhang J, Jin C, Liu Y, Luo W, Zheng W. Ionic liquid-controlled growth of NiCo2S4 3D hierarchical hollow nanoarrow arrays on Ni foam for superior performance binder free hybrid supercapacitors. Small. 2018;15(3):1804318.
Wang X, Xiao Y, Wang J, Sun L, Cao M. Facile fabrication of molybdenum dioxide/nitrogen-doped graphene hybrid as high performance anode material for lithium ion batteries. J Power Sour. 2015;274:142.
Li F, Rc Xu, Ym Li, Liang F, Zhang DF, Fu WF, Lv XJ. N-doped carbon coated NiCo2S4 hollow nanotube as bifunctional electrocatalyst for overall water splitting. Carbon. 2019;145:521.
Kang H, Li H, Zhao X, Yang L, Xu S. Anion doped bimetallic selenide as efficient electrocatalysts for oxygen evolution reaction. Ceram Int. 2020;46(3):2792.
Shuai C, Mo Z, Niu X, Yang X, Liu G, Wang J, Liu N, Guo R. Hierarchical NiCo2S4 nanosheets grown on graphene to catalyze the oxygen evolution reaction. J Mater Sci. 2019;55(4):1627.
Xu J, Rong J, Qiu F, Zhu Y, Mao K, Fang Y, Yang D, Zhang T. Highly dispersive NiCo2S4 nanoparticles anchored on nitrogen-doped carbon nanofibers for efficient hydrogen evolution reaction. J Colloid Interface Sci. 2019;555:294.
Xin W, Jiang WJ, Lian Y, Li H, Hong S, Xu S, Yan H, Hu JS. NiS2 nanodotted carnation-like CoS2 for enhanced electrocatalytic water splitting. Chem Commun. 2019;55(26):3781.
Hong X, Li S, Tang X, Sun Z, Li F. Self-supporting porous CoS2/rGO sulfur host prepared by bottom-up assembly for lithium-sulfur batteries. J Alloys Compd. 2018;749:586.
Bai D, Wang F, Lv J, Zhang F, Xu S. Triple-confined well-dispersed biactive NiCo2S4/Ni0.96S on graphene aerogel for high-efficiency lithium storage. ACS Appl Mater Interfaces. 2016;8(48):32853.
Wang J, Xu F, Jin H, Chen Y, Wang Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv Mater. 2017;29(14):1605838.
Zhou M, Weng Q, Zhang X, Wang X, Xue Y, Zeng X, Bando Y, Golberg D. In situ electrochemical formation of core-shell nickel-iron disulfide and oxyhydroxide heterostructured catalysts for a stable oxygen evolution reaction and the associated mechanisms. J Mater Chem A. 2017;5(9):4335.
Guo Y, Park T, Yi JW, Henzie J, Kim J, Wang Z, Jiang B, Bando Y, Sugahara Y, Tang J, Yamauchi Y. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv Mater. 2019;31(17):1807134.
Konkena B, Masa J, Botz AJR, Sinev I, Xia W, Koßmann J, Drautz R, Muhler M, Schuhmann W. Metallic NiPS3@NiOOH core–shell heterostructures as highly efficient and stable electrocatalyst for the oxygen evolution reaction. ACS Catal. 2016;7(1):229.
Huang L, Shin H, Goddard WA, Wang J. Photochemically deposited Ir-doped NiCo oxyhydroxide nanosheets provide highly efficient and stable electrocatalysts for the oxygen evolution reaction. Nano Energy. 2020;75:104885.
JingYang HL, Martens WN, Frost. RL. Synthesis and characterization of cobalt hydroxide cobalt oxyhydroxide and cobalt oxide nanodiscs. J Phys Chem C. 2010;114(1):111.
Wang R, Xu C, Lee JM. High performance asymmetric supercapacitors: new NiOOH nanosheet/graphene hydrogels and pure graphene hydrogels. Nano Energy. 2016;19:210.
Zou H, He B, Kuang P, Yu J, Fan K. Metal-organic framework-derived nickel-cobalt sulfide on ultrathin mxene nanosheets for electrocatalytic oxygen evolution. ACS Appl Mater Interfaces. 2018;10(26):22311.
Fan K, Zou H, Lu Y, Chen H, Li F, Liu J, Sun L, Tong L, Toney MF, Sui M, Yu J. Direct observation of structural evolution of metal chalcogenide in electrocatalytic water oxidation. ACS Nano. 2018;12(12):12369.
Li H, Xu SM, Li Y, Yan H, Xu S. An in situ phosphorization strategy towards doped Co2P scaffolded within echinus-like carbon for overall water splitting. Nanoscale. 2020;12(37):19253.
Chen P, Xu K, Fang Z, Tong Y, Wu J, Lu X, Peng X, Ding H, Wu C, Xie Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew Chem Int Ed Eng. 2015;54(49):14710.
Bora A, Mohan K, Doley S, Dolui SK. Flexible asymmetric supercapacitor based on functionalized reduced graphene oxide aerogels with wide working potential window. ACS Appl Mater Interfaces. 2018;10(9):7996.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.U1607128).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, D., Chang, YX., Li, YR. et al. Well-dispersed NiCoS2 nanoparticles/rGO composite with a large specific surface area as an oxygen evolution reaction electrocatalyst. Rare Met. 40, 3156–3165 (2021). https://doi.org/10.1007/s12598-021-01733-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01733-0