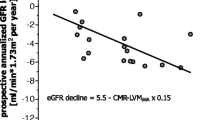
Abstract
Chronic kidney disease (CKD) is a global health problem and is independently associated with increased risk for cardiovascular disease (CVD). The presence and severity of CKD is strongly related to the progression of coronary atherosclerosis, ventricular hypertrophy, myocardial fibrosis, valvular calcification, and cardiac conduction system abnormalities. Echocardiography plays a major role in the assessment of structural and functional cardiac abnormalities in CKD including abnormal left-ventricular (LV) geometry, LV diastolic dysfunction, valvular disease, and left atrial dilatation, which are very frequently present especially in patients with end-stage renal disease.



Similar content being viewed by others
References
Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–30.
Krishnasamy R, Isbel NM, Hawley CM, et al. The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol Dial Transpl. 2014;29:1218–25.
Parikh NI, Hwang SJ, Larson MG, et al. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–911.
Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55.
Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–72.
Shlipak MG, Fried LF, Stehman-Breen C, et al. Chronic renal insufficiency and cardiovascular events in the elderly: findings from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:81–90.
van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352
Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–25.
Kadappu KK, Abhayaratna K, Boyd A, et al. Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiogr. 2016;29:359–67.
Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1-476.
McCullough PA. Cardiovascular disease in chronic kidney disease from a cardiologist's perspective. Curr Opin Nephrol Hypertens. 2004;13:591–600.
Pecoits-Filho R, Barberato SH. Echocardiography in chronic kidney disease: diagnostic and prognostic implications. Nephron Clin Pract. 2010;114:c242–c247247.
Chawla LS, Herzog CA, Costanzo MR, et al. Proposal for a functional classification system of heart failure in patients with end-stage renal disease: proceedings of the acute dialysis quality initiative (ADQI) XI workgroup. J Am Coll Cardiol. 2014;63:1246–52.
Hickson LJ, Negrotto SM, Onuigbo M, et al. Echocardiography Criteria for Structural Heart Disease in Patients With End-Stage Renal Disease Initiating Hemodialysis. J Am Coll Cardiol. 2016;67:1173–82.
Matsuo H, Dohi K, Machida H, et al. Echocardiographic assessment of cardiac structural and functional abnormalities in patients with end-stage renal disease receiving chronic hemodialysis. Circ J. 2018;82:586–95.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14.
Pluta A, Strozecki P, Krintus M, et al. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren Fail. 2015;37:1105–10.
Mark PB, Johnston N, Groenning BA, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839–45.
Stewart GA, Gansevoort RT, Mark PB, et al. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005;67:217–26.
Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–84.
Takeda A, Toda T, Iwamoto H, et al. Long-term evolution and changing associations of left ventricular hypertrophy after starting hemodialysis. Nephron Clin Pract. 2008;110:c126–c132132.
Hayashi T, Kimura T, Yasuda K, et al. Prognostic significance of left ventricular hypertrophy observed at dialysis initiation depends on the pre-dialysis use of erythropoiesis-stimulating agents. Clin Exp Nephrol. 2013;17:294–303.
Park M, Hsu CY, Li Y, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–34.
Charytan D. Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23:578–85.
Di Lullo L, Gorini A, Russo D, et al. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5:254–66.
Buckberg G, Hoffman JI, Mahajan A, et al. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87.
Krishnasamy R, Isbel NM, Hawley CM, et al. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS ONE. 2015;10:e0127044.
Liu YW, Su CT, Huang YY, et al. Left ventricular systolic strain in chronic kidney disease and hemodialysis patients. Am J Nephrol. 2011;33:84–90.
Wang H, Liu J, Yao XD, et al. Multidirectional myocardial systolic function in hemodialysis patients with preserved left ventricular ejection fraction and different left ventricular geometry. Nephrol Dial Transpl. 2012;27:4422–9.
Hayashi SY, Seeberger A, Lind B, et al. A single session of haemodialysis improves left ventricular synchronicity in patients with end-stage renal disease: a pilot tissue synchronization imaging study. Nephrol Dial Transpl. 2008;23:3622–8.
Shi F, Feng S, Zhu J, et al. Left ventricular strain and dyssynchrony in young and middle-aged peritoneal dialysis patients and healthy controls: a case-matched study. Cardiorenal Med. 2018;8:271–84.
Takahashi N, Sato N, Ishikawa M, et al. Long-term hemodialysis corrects left ventricular dyssynchrony in end-stage renal disease: a study with gated Technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Nippon Med Sch. 2015;82:76–83 (Erratum in 2015; 82:166)
Murata T, Dohi K, Onishi K, et al. Role of haemodialytic therapy on left ventricular mechanical dyssynchrony in patients with end-stage renal disease quantified by speckle-tracking strain imaging. Nephrol Dial Transpl. 2011;26:1655–61.
Smiseth OA. Evaluation of left ventricular diastolic function: state of the art after 35 years with Doppler assessment. J Echocardiogr. 2018;16:55–64.
Takamura T, Dohi K, Onishi K, et al. Left ventricular contraction-relaxation coupling in normal, hypertrophic, and failing myocardium quantified by speckle-tracking global strain and strain rate imaging. J Am Soc Echocardiogr. 2010;23:747–54.
Vogel MW, Slusser JP, Hodge DO, et al. The natural history of preclinical diastolic dysfunction: a population-based study. Circ Heart Fail. 2012;5:144–51.
Farshid A, Pathak R, Shadbolt B, et al. Diastolic function is a strong predictor of mortality in patients with chronic kidney disease. BMC Nephrol. 2013;14:280.
Sharma R, Pellerin D, Gaze DC, et al. Mitral peak Doppler E-wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end-stage renal disease. J Am Soc Echocardiogr. 2006;19:266–73.
Wang AY, Wang M, Lam CW, et al. Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension. 2008;52:107–14.
Purga SL, Karas MG, Horn EM, et al. Contribution of the left atrial remodeling to the elevated pulmonary capillary wedge pressure in patients with WHO Group II pulmonary hypertension. J Echocardiography. 2018 (Epub ahead of print).
Tripepi G, Benedetto FA, Mallamaci F, et al. Left atrial volume in end-stage renal disease: a prospective cohort study. J Hypertens. 2006;24:1173–80.
Tripepi G, Benedetto FA, Mallamaci F, et al. Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: a prospective cohort study. J Am Soc Nephrol. 2007;18:1316–22.
Tripepi G, Mattace-Raso F, Mallamaci F, et al. Biomarkers of left atrial volume: a longitudinal study in patients with end stage renal disease. Hypertension. 2009;54:818–24.
Han JH, Han JS, Kim EJ, et al. Diastolic dysfunction is an independent predictor of cardiovascular events in incident dialysis patients with preserved systolic function. PLoS ONE. 2015;10:e0118694.
Maher ER, Young G, Smyth-Walsh B, et al. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;2:875–7.
Ribeiro S, Ramos A, Brandao A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transpl. 1998;13:2037–40.
Rattazzi M, Bertacco E, Del Vecchio A, et al. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transpl. 2013;28:2968–76.
Ohara T, Hashimoto Y, Matsumura A, et al. Accelerated progression and morbidity in patients with aortic stenosis on chronic dialysis. Circ J. 2005;69:1535–9.
Elgendy IY, Conti CR. Caseous calcification of the mitral annulus: a review. Clin Cardiol. 2013;36:E27–31.
Garcia-Ibarrondo N, Lang RM. Caseous calcification of the mitral annulus, a rare echocardiographic finding. Rev Esp Cardiol. 2011;64:828–31 (Article in Spanish)
Takeuchi T, Dohi K, Sato Y, et al. Calcified amorphous tumor of the heart in a hemodialysis patient. Echocardiography. 2016;33:1926–8.
Honda S, Kawasaki T, Yamano M, et al. A case of calcified amorphous tumor with caseous calcification of the mitral annulus. Jpn J Med Ultrasonics. 2016;43:577–80 (Article in Japanease)
Katzarski KS, Randmaa I, Bergstrom J. Influence of hemodialysis on intravascular volume and vasoactive hormones. Clin Nephrol. 1999;52:304–11.
Sabaghian T, Hajibaratali B, Samavat S. Which echocardiographic parameter is a better marker of volume status in hemodialysis patients? Ren Fail. 2016;38:1659–64.
Cristina Di Gioia M, Gascuena R, Gallar P, et al. Echocardiographic findings in haemodialysis patients according to their state of hydration. Nefrologia. 2017;37:47–53.
Mavrakanas TA, Khattak A, Singh K, et al. Echocardiographic parameters and renal outcomes in patients with preserved renal function, and mild- moderate CKD. BMC Nephrol. 2018;19:176.
Sugiura E, Dohi K, Onishi K, et al. Reversible right ventricular regional non-uniformity quantified by speckle-tracking strain imaging in patients with acute pulmonary thromboembolism. J Am Soc Echocardiogr. 2009;22:1353–9.
Atsumi A, Seo Y, Ishizu T, et al. Right ventricular deformation analyses using a three-dimensional speckle-tracking echocardiographic system specialized for the right ventricle. J Am Soc Echocardiogr. 2016;29(402–11):e2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.D. received lecture fees of equal to or more than 500,000 yen from Otsuka Pharma Inc. and Takeda Pharmaceutical Co. Ltd. in 2018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dohi, K. Echocardiographic assessment of cardiac structure and function in chronic renal disease. J Echocardiogr 17, 115–122 (2019). https://doi.org/10.1007/s12574-019-00436-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-019-00436-x