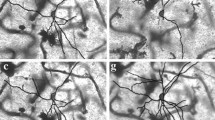
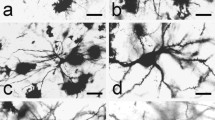
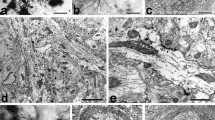
Abstract
A qualitative analysis of the morphology of human putamen nerve cells involves a detailed description of the structure and features of neurons and, accordingly, their classification into already defined classes and types. In our sample of 301 neurons, 64.78 % (195) were spiny and 35.22 % (106) aspiny cells. By analyzing cell bodies and dendritic trees, we subdivided spiny cells into two types (I and II) and aspiny cells into three types (III, IV and V). Our sample of neurons, classified according to the previously described scheme, consisted of 80 type I, 115 type II, 16 type III, 42 type IV and 48 type V nerve cells. In the present study, after qualitative analysis of microscopic images of the Golgi impregnated neurons of the putamen, we measured/quantified five morphological properties, i.e., the sizes of the soma and dendritic field, shape of the neuron, straightness of individual dendrites and the branching complexity of the dendritic tree, using eight morphometric parameters. Hence, we identify five types of nerve cells in the human putamen: type I—small spiny neurons; type II—large spiny neurons; type III—large aspiny neurons; type IV—neurons with a large soma and a medium dendritic field; and type V—small aspiny neurons. By performing an adequate statistical analysis on these parameters, we point out that the proposed types differ enough in their morphology to warrant our qualitative classification.





Similar content being viewed by others
References
Adinofi AM, Pappas GD (1968) The fine structure of the caudate nucleus of the cat. J Comp Neurol 133:168–184
Bernácer J, Prensa L, Giménez-Amaya JM (2007) Cholinergic interneurons are differentially distributed in the human striatum. PLoS One 2(11):e1174
Bernácer J, Prensa L, Giménez-Amaya JM (2008) Chemical architecture of the posterior striatum in the human brain. J Neurol Transm 115:67–75
Braak H, Braak E (1982) Neuronal types in the striatum of man. Cell Tissue Res 227:319–342
Chang HT, Wilson CJ, Kitai ST (1982) A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol 208:107–126
Chesselet MF, Plotkin JL, Wu N, Levine MS (2007) Development of striatal fast-spiking GABAergic interneurons. Prog Brain Res 160:261–272
DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64:20–24
DiFiglia M, Pasik P, Pasik T (1976) A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res 114:245–256
DiFiglia M, Pasik T, Pasik P (1980) Ultrastructure of Golgi-impregnated and gold-toned spiny and aspiny neurons in the monkey neostriatum. J Neurocytol 9:471–492
Dimova R, Vuillet J, Seite R (1980) Study of the rat neostriatum using a combined Golgi-electron microscope technique and serial sections. Neuroscience 5:1581–1596
Fernández E, Jelinek HF (2001) Use of fractal theory in neuroscience: methods, advantages, and potential problems. Methods 24:309–321
Fino E, Venance L (2011) Spike-timing dependent plasticity in striatal interneurons. Neuropharmacology 60:780–788
Ghiglieri V, Bagetta V, Calabresi P, Picconi B (2011) Functional interactions within striatal microcircuit in animal models of Huntington’s disease. Neuroscience. doi:10.1016/j.neuroscience.2011.06.075
Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234
Graveland GA, DiFiglia M (1985) The frequency and distribution of medium-sized neurons indented nuclei in the primate and rodent neostriatum. Brain Res 327:307–311
Graveland GA, Williams RS, DiFiglia M (1985) A Golgi study of the human neostriatum: neurons and afferent fibers. J Comp Neurol 234:317–333
Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26:317–330
Hisano S (2003) Vesicular glutamate transporters in the brain. Anat Sci Int 78:191–204
Kemp JM, Powell TPS (1971) The structure of caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond (Biol) 262:383–401
Kuricky BY (1969) Mathematical methods in physiology (in Russian). Nauka, Leningrad
Lalošević D, Somer Lj, Đolai M, Lalošević V, Mažibrada J, Krnojelac D (2005) Mikroskopska laboratorijskatehnika u medicini. Medicinski fakultet, Novi Sad
Leontovich TA (1998) Large neostriatal neurons in humans and their possible role in neuronal networks. Neurosci Behav Physiol 28:252–259
McNeill TH, Koek LL (1990) Differential effects of advancing age on neurotransmitter cell loss in the substantia nigra and striatum of C57BL/6N mice. Brain Res 521:107–117
Mellios K, Zacharaki T, Sophou S, Latsari M, Antonopoulos J, Dinopoulos A, Parnavelas JG, Dori I (2009) Natural and lesion-induced apoptosis in the rat striatum during development. Brain Res 1252:30–44
Milošević NT, Ristanović D, Jelinek HF, Rajković K (2009) Quantitative analysis of dendritic morphology of the alpha and delta retinal ganglion cells in the rat: a cell classification study. J Theor Biol 259:142–150
Milošević NT, Krstonošić B, Ristanović D, Gudović R (2011) Fractal analysis of neuronal dendritic branching pattern in the human neostriatum: a revised classification scheme. In: Dobrescu R (ed) Proceedings CSCS-18. Interdisciplinary approaches in fractal analysis IAFA 2011, vol 3. Editura Politehnica, Bucharest, pp 871–876
Parent A (1990) Extrinsic connections of the basal ganglia. Trends Neurosci 13:254–258
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Ramanathan S, Hanley JJ, Deniau JM, Bolam JP (2002) Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci 22:8158–8169
Raz N, Torres IJ, Acker JD (1995) Age, gender and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiol Learn Mem 63:133–142
Ristanović D, Milošević NT, Jelinek HF, Stefanović IB (2009a) Mathematical modelling of neuronal dendritic branching patterns in two dimensions: application to retinal ganglion cells in the cat and rat. Biol Cybern 100:97–108
Ristanović D, Milošević NT, Stefanović IB, Marić D, Popov I (2009b) Cell image area as a tool for neuronal classification. J Neurosci Methods 182:272–278
Ristanović D, Milošević NT, Stefanović BD, Marić DL, Rajković K (2010) Morphology and classification of large neurons in the adult human dentate nucleus: a qualitative and quantitative analysis of 2D images. Neurosci Res 67:1–7
Ristanović D, Milošević NT, Štulić V (2006) Application of modified Sholl analysis to neuronal dendritic arborization of the cat spinal cord. J Neurosci Methods 158:212–218
Schmitt O, Eggers R, Haug H (1995) Quantitative investigations into the histostructural nature of the human putamen I. Staining, cell classification and morphometry. Ann Anat 177:243–250
Schröder KF, Hopf A, Lange H, Thörner G (1975) Morphometrisch-statistische Strukturanalysen des Striatum, Pallidum und Nucleus Subthalamicus beim Menschen. J Hirnforsch 16:333–350
Somogy JP, Bolam JP, Smith AD (1981) Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscope study using the Golgi-peroxidase transport degeneration procedure. J Comp Neurol 195:567–584
Steiner H, Tseng KY (2010) Handbook of basal ganglia structure and function. Academic, London
Tanaka D Jr (1980) Development of spiny and aspiny neurons in the caudate nucleus of the dog during the first postnatal month. J Comp Neurol 192:247–264
Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692
Wilson CJ, Groves PM, Kitai ST, Linder JC (1983) Three-dimensional structure of dendritic spines in the rat neostriatum. J Neurosci 3:383–398
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res 863:182–191
Yelnik J, François C, Percheron G, Tandé D (1991) Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol 313:273–294
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krstonošić, B., Milošević, N.T., Gudović, R. et al. Neuronal images of the putamen in the adult human neostriatum: a revised classification supported by a qualitative and quantitative analysis. Anat Sci Int 87, 115–125 (2012). https://doi.org/10.1007/s12565-012-0131-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-012-0131-4