Abstract
Purpose
In the present brief report, the authors summarized the data on the use of carbon ion radiotherapy in gynaecological malignancies starting from the preclinical evidence to move forward the clinically available literature and hence focusing on the possible future application directions.
Methods
This is a short report of the published studies on the role of carbon ion radiotherapy in the treatment of gynaecological malignancies.
Results
The use of carbon ion radiotherapy in rare and radioresistant gynaecological tumors is supported by preclinical and clinical data. In particular, carbon ion radiotherapy appears to be safe and effective in the management of cervical adenocarcinomas, unresectable endometrial cancers, mucosal melanomas of the lower genital tract and vulvar adenoid cystic carcinomas. Moreover, considering the dosimetric characteristics, carbon ions are suitable for recurrent disease in the pelvis. Prospective and phase II trials on gynaecological melanomas and pelvic relapses after radiotherapy are currently ongoing. The future study directions might be the oligometastatic diseases and the oncofertility.
Conclusions
More and more growing preclinical and clinical evidence supports the use of carbon ion radiotherapy in gynaecological oncology. Strong and multidisciplinary collaborations at national and international levels are desirable to better understand the therapeutic and organizational benefits of this new technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Due to the favourable dose profile and the advantageous radiobiological effects over photon beam radiotherapy (X-RT), carbon ion radiotherapy (CIRT) has gained increasing oncological favour for the treatment of radioresistant histologies and tumours located close to high radiosensitivity organs at risk [1]. These edges, proved both in preclinical and clinical experiences, provide a strong rationale for the application of CIRT in several oncological settings [2], especially in head and neck radioresistant malignancies [3,4,5,6,7] and in bone and soft tissue sarcomas [8,9,10,11,12].
However, in recent years accumulating evidence has demonstrated a promising application of CIRT in gynaecological oncology [13]. Herein we briefly summarized the current preclinical and clinical evidence on CIRT for the treatment of gynaecological tumors. We also speculated about the possible future direction, emphasizing the potentiality concerning the ballistic hallmarks of CIRT, the superior radiobiological features along with the molecular background of the tumours.
2 Radiobiological effect of carbon ion radiotherapy in gynaecological tumours
2.1 CIRT overcomes the radioresistance of cervical cancer and vaginal malignant melanomas
Quiescent cells (G0) are in a sleep-like cellular status, and have a higher hypoxic proportion and better capacity for repair [14]. When compared to X-RT, CIRT was shown to be most efficient in eliminating G0 cancer cells [15]. Among the strategies reported in the literature to promote this effect [1], in vitro studies showed that CIRT mainly acted on adenocarcinoma cervical cancer cell line (HeLa) in three ways: (i) induction of the apoptosis of G0 cells via an enhanced mitochondria-mediated intrinsic pathway; (ii) direct cell death by clustered DNA damages; (iii) induction a cell cycle re-entry and G2/M arrest of the quiescent cells . Interestingly all these phenomena to overcome HeLa radioresistance seemed to be mediated mainly by the Wnt/β‐catenin signaling [16, 17]. Wnt/β-catenin signaling is involved in several essential biological processes, including tissue homeostasis, stem cell regrowth, and cell survival and it is reported as crucial for preserving quiescent cells’ latent condition [18,19,20]. In vitro experiments proved that after CIRT the expression of Wnt3a and β‐catenin significantly decreased in proliferating HeLa cells while increasing in G0 cells, suggesting that CIRT might face the radioresistance of this cytotype by the inhibition of this pathway [15, 17]. Moreover, due to the down-regulation of Wnt/β-catenin, there was a significant reduction of DNA damage, a consistent improvement of DNA damage repair, maintenance of G0 status and a decreased apoptosis [15].
Compared to X-RT and consistently to HeLa cell lines, CIRT resulted in a larger ratio of necrosis to apoptosis and a stronger inhibition of cell growth in melanoma cell lines. Furthermore, CIRT showed a longer-lasting and greater G2/M arrest by activation of the pRb/E2F1/Chk2 pathway [21]. Moreover, CIRT has proved to decrease cell viability, proliferation, and migration of a cell line of vaginal melanoma (HMV-II) [22], and to activate their dendricity [2] also through the modulation of Ca 2 + signaling [23]. Figure 1 synthetized the above-reported data.
Mechanisms of CIRT overcoming the radioresistance of cervical cancer and vaginal malignant melanomas [74]
2.2 CIRT triggers the tumour immune modulation in cervical cancer and malignant melanomas
High Mobility Group Box 1 (HMGB1) is one of the main damage-associated molecular signals able to enhance the anti-tumour adaptive immunity after radiotherapy (RT) exposure [24]. Considering that it is a nuclear chromatin-associated non-histone protein, the complex double-strand breaks (DSBs) DNA damages induced by CIRT lead to a significant release of HMGB1 from cancer cells over X-RT [25] when administered at iso-survival doses [26]. Indeed, in their in vitro study, Onishi et al. [26] demonstrated that CIRT significantly increased HMGB1 levels in the culture supernatants of HeLa and squamous cervical carcinoma SiHa cell lines with increased LET, suggesting the ability to trigger a more efficient anti-tumour immunity than X-RT. Moreover, the in vitro study by Iijima et al. [27] showed that CIRT, compared to X-RT, significantly upregulated the expression of programmed cell death-ligand 1 (PD-L1) in a dose-dependent manner in HeLa and SiHa cells through the phosphorylation of Chk1 pathway mediated by the DNA DSBs. These results were confirmed also in vivo by the same group. Indeed, the authors collected biopsy specimens of 33 cases of cervical adenocarcinomas before and after 12 Gy[RBE] of CIRT finding that the expression of PD-L1 was significantly increased after CIRT (p = 0.046) and in 8 cases of PD-L1 negative before irradiation there was a conversion into a positive status after 12 Gy[RBE]. Although the local control (LC), the progression-free survival (PFS), and overall survival (OS) were unrelated to the baseline PD-L1 status, the PD-L1 positivity after CIRT seemed to be advantageous in terms of PFS.
Consistently to cervical adenocarcinoma data, in a murine model of malignant melanomas, CIRT has proved to trigger immunogenic death, increasing the tumour immunogenicity and enhancing the response to sequential immunotherapy more efficiently than X-RT. The via used by CIRT and anti-PD-1 included the release of adenosine triphosphate (ATP), the release of HMGB1, the exposure of calreticulin, and the production of type-1 interferon responses. Furthermore, CIRT combined with anti-PD-1 boosted the number of CD4 + and CD8 + lymphocytes infiltrating the tumour bed, which considerably slowed the development of the tumour and extended the survival of mice with melanoma [28].
3 Carbon ion radiotherapy in gynecological malignancies: clinical evidence
CIRT data in gynaecological oncology mainly concerned uterine cervical carcinomas followed by endometrial carcinomas, rare tumours (i.e. malignant mucosal melanomas of the low genital tract and adenoid cystic carcinomas) and pelvic recurrences after X-RT.
3.1 Uterine cervical cancer
CIRT for cervical cancers was implemented at the National Institute of Radiological Sciences (NIRS) of Chiba in Japan in 1995 with consecutive dose-escalation studies that proved the feasibility and the effectiveness of a whole pelvis CIRT in this setting. In their systematic review of the literature, collecting data up to December 2018, Wang et al. reported for locally advanced cervical cancers treated with CIRT a local control (LC) ranging between 60.1 and 83.6% at 2 years to 53-83.6% at 5 years, despite unsatisfactory controls on the para-aortic lymph node recurrences [13]. The implementation of a prophylactic extended-field (para-aortic nodal) irradiation (protocols: 9702, 9902 and 0508) and the administration of concomitant chemotherapy (protocol 1001) reduced the risk of lymph nodal failures, with no increased toxicity [13, 29,30,31].
Going forward 2018, the deadline of this systematic review, the newest data on CIRT in cervical cancer become more and more intriguing, especially for the adenocarcinomas, the most radioresistant histology. Indeed, the propensity score-matched analysis demonstrated that for adenocarcinomas, the combined approach with weekly Cisplatin improved the long-term overall survival- OS- ( 72% vs. 46% at 5 years, p = 0.041) and the 5-year progression-free survival -PFS- ( 66% vs. 41% p = 0.048) but did not impact the LC (53% vs. 49% at 5-years, p = 0.086). These results suggested that CIRT did not need radiosensitizing to increase its local effect [32]. These data are encouraging the use of chemo-CIRT in cervical adenocarcinomas, considering that the reported 5-year OS of the X-RT historical cohort was up to 33% [33,34,35,36]. The effectiveness of CIRT in this histology was also proved by a multi-institutional study on 55 cases of locally advanced cervical adenocarcinomas (IIB–IVA ) in which the 5-year OS and 5-year LC rates were 68.6% and 65.2%, respectively, with no significant impact of the concomitant chemotherapy [37]. Moreover, the authors identified FIGO staging (5-year OS: IIB versus IIIB–IVA, 75.4% versus 54.3%, p = 0.019) and initial tumour response (5-year LC: p = 0.003, hazard ratio [HR]: 0.227) as predictive outcome factors. In both studies, the combined approach was safe with no differences in terms of haematological and non-haematological toxicities between CIRT alone and chemo-CIRT [32, 37]. Notably, also CIRT followed by brachytherapy has been reported to be safe [37, 38], paving the way to further investigations.
Considering the immunomodulation of CIRT in HeLa and SiHa cell lines, the radiation community is thrilled to wait for the final results of the DECISION trial, a phase I study aimed at evaluating the safety of a combination of CIRT with Durvalumab (humanized anti-PD-L1 monoclonal antibody) in patients with locally advanced cervical cancer. The preliminary report on the first 3 patients enrolled suggested the effectiveness with a total of 100% of complete responses. Concerning safety, authors described grade 3 neutropenia (100%), grade 3 increased gamma-glutamyl transpeptidase levels (33%) and grade 3 hypothyroidism (33%) [39].
3.2 Gynecological melanomas
Among melanomas, gynaecological mucosal melanomas are the rarest form with the most dismal prognosis and higher radioresistance features. To date, there are no guidelines or consensus about the management of these challenging diseases, but the therapeutic approaches are based on data concerning skin melanomas and other gynaecological histologies [40, 41]. Considering the radioresistance characteristics of the disease, mucosal melanomas are ideal for testing the efficacy of CIRT.
The largest series on the use of radical CIRT in the treatment of unresectable malignant mucosal melanomas was published by Murata et al [42]. Thirty-seven patients, 9 of whom with post-surgical recurrence, with vaginal (N = 22), vulvar (N = 12) and cervical (N = 3) tumours were treated up to a total dose of 57.6 GyRBE in 16 fractions (N = 35) or 64.0 GyE in 16 fractions (N = 2). 81% experienced a complete response within 6 months, with 2-year LC, OS, and PFS rates were 71% (CI: 53.6–87.6%), 53% (CI: 36.3–69.2%), and 29% (CI: 14.0–4.7%), respectively. The combination of CIRT with immune checkpoint inhibitors appeared to be safe [43]. Considering these promising results along with the Italian preliminary experience [43,44,45], the National Center for Oncological Hadrontherapy (CNAO) activated a currently recruiting phase II prospective clinical trial (NCT05478876) to confirm in a prospective cohort the efficacy and feasibility of CIRT in unresectable gynaecological melanomas.
3.3 Unresectable endometrial cancers
The most frequent gynaecological malignancy in high-income countries is endometrial cancer, which ranks second in low-income ones after cervical cancer. Obesity and diabetes, particularly common in the ever-ageing population of Western countries, are often associated with cardiovascular diseases and overall contraindicated surgery in up to 9% of the cases [46]. In case of unresectability, radical RT with or without brachytherapy might be an option. Considering the radioresistance of the histology, and the increased percentage of women unfit for surgery in developed nations, endometrial adenocarcinomas appear suitable to assess the efficacy of CIRT. To the best of our knowledge, the only experience testing radical CIRT for inoperable endometrial adenocarcinomas was the pooled analysis of 14 cases by Irie et al. [47]. These women underwent radical CIRT ( 62.4–74.4 GyRBE in 20 fractions) for tumors at stage I-III achieving a high objective response rate (10 complete responses, 3 partial responses and 1 stable disease), with a 5-year LC of 86%(95% CI: 67–100%), and no grade ≥ 3 acute or late toxicity, really promising results compared to X-RT data [48,49,50].
3.4 Adenoid cystic carcinomas
Adenoid cystic carcinoma (ACC) is a rare disease that often develops from the salivary glands in the head and neck area (age-adjusted incidence rate: 4.5 occurrences per 100,000), and barely in other anatomical sites such as breast, lung and vulva where ACCs arise from seromucous glandular structures. Vulvar ACCs are extremely rare, with only approximately 350 cases reported in the literature, representing less than 1% of all vulvar malignancies [51]. For their marked neurotropism and pronounced radioresistance, this histology is an ideal candidate to assess the feasibility of CIRT and, actually, several data on head and neck cancers are encouraging [52]. Using a mixed beam approach (CIRT first followed by X-RT), a German study revealed safe and successful outcomes in pelvic ACCs [53]. The authors described the results of X-RT of the lymph node drainage up to 50 Gy, using helical tomotherapy, in three cases of recurrent pelvic ACC, one of which from the Bartholin’s gland after radical surgery, preceded by a CIRT boost on macroscopic tumour up to a total dose of 24 GyRBE. The patient achieved a partial remission which persisted for 16 months after CIRT.
3.5 Re-irradiation of gynaecological pelvic relapses
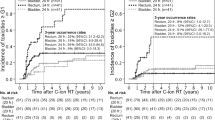
Considering the ballistic hallmarks, re-irradiation of a recurrence within or at the edge of a previous X-RT field is one of the cornerstones of CIRT corroborated by several data on the safety and efficacy for different anatomical sites and on several histologies. It is not an exception the gynaecological oncology in which CIRT up to a total dose of 48-57.6 GyRBE (in 12 or 16 fractions) achieved long-term LC when delivered to unresectable pelvic failures after a previous pelvic X-RT [54]. Indeed, the 3-year LC was 94% (95% CI, 66–99%) on 16 analyzed cases and, compared to X-RT series (in which toxicities higher or equal to grades 3–4 were up to 26% after brachytherapy and 20% after stereotactic RT [55]), no grade ≥ 3 toxicities were recorded. Due to the promising result of this Japanese cohort and the preliminary Italian data [56], CNAO started a phase II clinical study, currently recruiting, on reirradiation of gynaecological recurrences (NCT05457595) with LC, defined as the absence of local progression at 1 year after treatment, as the primary endpoint. The trial, currently open to recruitment, would assess the effectiveness and safety of this approach in a prospective cohort.
4 Future perspectives
Growing data backs up the concept that oligometastases are a cancer state in between a localized tumour and a systemic spread disease, where localized treatments are able to extend survival. Hypofractionated X-RT was safe and provided durable LC when delivered for oligometastatic and oligopersistant gynaecological disease [57]. Indeed, MITO-RT1 and MITO-RT2/RAD studies achieved a 2-year actuarial LC rate of 81.9% in oligometastatic ovarian and 75% in oligometastatic uterine cancers [58, 59]. These data suggested RT as a valid therapeutic option to prolong the chemotherapy rechallenge and to improve survival. Although the excellent physical characteristics and the greater radiobiological potential, especially for hypoxic, radioresistant tumours, as well as homologous recombination deficiency and wild-type BRCA tumours [1, 60,61,62,63,64,65], there is still limited evidence about CIRT in the management of oligometastatic disease. In this challenging scenario, a recent nationwide multi-institutional cohort study explored the role of particle beam RT ( proton and CIRT) in the management of 614 patients ( for a total of 841 oligometastases), including 13 cases of ovarian and 45 cases of uterine cancers [66]. This large cohort study showed an overall durable LC (72.8–83.2% at 3 years), superior to stereotactic RT in the case of the liver (incidence rate ratio [IRR], 0.52 ;95% CI, 0.37–0.72; P = < 0.001). and lung metastases (IRR, 0.56; 95% CI, 0.34–0.91, P = 0.020) and similar for lymph node ones ( IRR,0.82; 95% CI, 0.61–1.11; P = 0.200), with an acceptable risk of severe toxicity (0.8–3.5%). Overall these results suggested that particle beam RT may be a new treatment option in the management of these diseases. CNAO and the National Institutes for Quantum and Radiological Science and Technology (QST) of Chiba conducted a bi-institutional study, whose preliminary results were recently presented at the last ESTRO meeting, to evaluate for the first time in literature the use of radical CIRT in oligometastatic ovarian cancers [67]. The data were encouraging in terms of objective response rate ( 85%) and LC ( 89% and 74% at 1 and 2 years) along with toxicity ( only one case of grade 3 gastro-intestinal toxicity), with a safety profile also in patients receiving PARP-I and anti-VEGF [67]. Considering that all efforts should be made to avoid a platinum rechallenge in this PARP-I era, a better selection of ovarian cancer patients to treat with stereotactic RT vs. CIRT should take into consideration the tumour molecular profile and the radiobiological characteristics of these approaches. The effect of the combination with new drugs such as PARP-I should be evaluated.
Furthermore, considering the preclinical evidence about the immunomodulation of CIRT in gynaecological tumours, possible future strategies should test the combination between immunotherapy and CIRT in gynaecological radioresistant diseases (i.e. cervical adenocarcinomas and gynaecological melanomas) to confirm the safety profile of the combination and to evaluate the potentiality of CIRT to reverse desert and cold malignancies into immune-responsive ones [68,69,70,71].
Moreover, because of the advantageous dose deposition of CIRT, this technique is potentially feasible in the fertility-sparing management of young patients treated on the pelvis. This topic has become more and more crucial in recent years due to the rise in the average age of the first pregnancy. The high radiosensitivity of ovaries and the consequences on pregnancy after uterine irradiation should be considered during a pelvic RT and the patients should be accurately consulted about the fertility-sparing options. In this context, multidisciplinary management including prophylactic surgery to spare ovaries and uterine structures appeared safe and feasible [72] also after a high dose of CIRT for radioresistant histologies [73]. A multi-institutional registry cumulating data on the oncofertility potential of CIRT is warranted to collect more evidence.
Because of the complexity and the rare types of cancers treated with CIRT, the creation of national and international networks including research groups and experts in particle beam RT is desirable to create more robust evidence and to reduce barriers, facilitating the access to new RT beams.
5 Conclusions
CIRT appeared safe and effective in the treatment of radioresistant and difficult-to-cure gynaecological malignancies. The rationale for its use is supported by more and more growing preclinical and clinical evidence. The therapeutic and organizational benefits of this new technology in gynaecological oncology can only be fully realized with a thorough, mature, and even more strong multidisciplinary approach.
Data availability
No new data were generated or analyzed in this manuscript.
References
Tinganelli W, Durante M. Carbon Ion Radiobiology. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12103022.
Orlandi E, Barcellini A, Vischioni B, Fiore MR, Vitolo V, Iannalfi A, et al. The role of Carbon Ion Therapy in the changing Oncology Landscape-A Narrative Review of the literature and the Decade of Carbon Ion experience at the Italian National Center for Oncological Hadrontherapy. Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15205068.
Ronchi S, Cicchetti A, Bonora M, Ingargiola R, Camarda AM, Russo S, et al. Curative carbon ion radiotherapy in a head and neck mucosal melanoma series: facing the future within multidisciplinarity. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2023;190:110003. https://doi.org/10.1016/j.radonc.2023.110003.
Ikawa H, Koto M, Hayashi K, Tonogi M, Takagi R, Nomura T, et al. Feasibility of carbon-ion radiotherapy for oral non-squamous cell carcinomas. Head Neck. 2019;41:1795–803. https://doi.org/10.1002/hed.25618.
Mizoe J-E, Hasegawa A, Jingu K, Takagi R, Bessyo H, Morikawa T, et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2012;103:32–7. https://doi.org/10.1016/j.radonc.2011.12.013.
Vischioni B, Bonora M, Ronchi S, Ingargiola R, Camarda AM, Motinetli S, et al. OC-0110 Head and neck adenoid cystic carcinoma treated with raster scanning carbon ion radiotherapy at CNAO. Radiother Oncol. 2023;182:S70–1. https://doi.org/10.1016/S0167-8140(23)08524-9.
Akbaba S, Lang K, Held T, Bulut OC, Mattke M, Uhl M, et al. Accelerated hypofractionated active raster-scanned Carbon Ion Radiotherapy (CIRT) for laryngeal malignancies: feasibility and safety. Cancers (Basel). 2018;10. https://doi.org/10.3390/cancers10100388.
Imai R, Kamada T, Araki N. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018;7:4308–14. https://doi.org/10.1002/cam4.1679.
Demizu Y, Jin D, Sulaiman NS, Nagano F, Terashima K, Tokumaru S, et al. Particle therapy using protons or Carbon ions for Unresectable or incompletely resected bone and soft tissue sarcomas of the Pelvis. Int J Radiat Oncol Biol Phys. 2017;98:367–74. https://doi.org/10.1016/j.ijrobp.2017.02.030.
Cuccia F, Fiore MR, Barcellini A, Iannalfi A, Vischioni B, Ronchi S, et al. Outcome and Toxicity of Carbon Ion Radiotherapy for Axial Bone and Soft tissue sarcomas. Anticancer Res. 2020;40. https://doi.org/10.21873/anticanres.14260.
Mattke M, Ohlinger M, Bougatf N, Harrabi S, Wolf R, Seidensaal K, et al. Proton and carbon ion beam treatment with active raster scanning method in 147 patients with skull base chordoma at the Heidelberg Ion Beam Therapy Center-a single-center experience. Strahlentherapie Und Onkol Organ Der Dtsch Rontgengesellschaft [et Al]. 2023;199:160–8. https://doi.org/10.1007/s00066-022-02002-4.
Iannalfi A, D’Ippolito E, Riva G, Molinelli S, Gandini S, Viselner G, et al. Proton and carbon ion radiotherapy in skull base chordomas: a prospective study based on a dual particle and a patient-customized treatment strategy. Neuro Oncol. 2020;22. https://doi.org/10.1093/neuonc/noaa067.
Wang L, Wang X, Zhang Q, Ran J, Geng Y, Feng S, et al. Is there a role for carbon therapy in the treatment of gynecological carcinomas? A systematic review. Future Oncol. 2019;15:3081–95. https://doi.org/10.2217/fon-2019-0187.
Zhang J, Si J, Gan L, Di C, Xie Y, Sun C, et al. Research progress on therapeutic targeting of quiescent cancer cells. Artif Cells Nanomed Biotechnol. 2019;47:2810–20. https://doi.org/10.1080/21691401.2019.1638793.
Zhang J, Xie Y, Liu X, Gan L, Li P, Dou Z, et al. Carbon ions trigger DNA damage response to overcome radioresistance by regulating β-catenin signaling in quiescent HeLa cells. J Cell Physiol. 2023;238:1836–49. https://doi.org/10.1002/jcp.31052.
Yao G, Tang J, Yang X, Zhao Y, Zhou R, Meng R, et al. Cyclin K interacts with β-catenin to induce cyclin D1 expression and facilitates tumorigenesis and radioresistance in lung cancer. Theranostics. 2020;10:11144–58. https://doi.org/10.7150/thno.42578.
Zhang J, Si J, Gan L, Guo M, Yan J, Chen Y, et al. Inhibition of wnt signalling pathway by XAV939 enhances radiosensitivity in human cervical cancer HeLa cells. Artif Cells Nanomed Biotechnol. 2020;48:479–87. https://doi.org/10.1080/21691401.2020.1716779.
Ge Y-X, Wang C-H, Hu F-Y, Pan L-X, Min J, Niu K-Y, et al. New advances of TMEM88 in cancer initiation and progression, with special emphasis on wnt signaling pathway. J Cell Physiol. 2018;233:79–87. https://doi.org/10.1002/jcp.25853.
Yang G, Shen T, Yi X, Zhang Z, Tang C, Wang L, et al. Crosstalk between long non-coding RNAs and Wnt/β-catenin signalling in cancer. J Cell Mol Med. 2018;22:2062–70. https://doi.org/10.1111/jcmm.13522.
Jing Q, Li G, Chen X, Liu C, Lu S, Zheng H, et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J Cell Mol Med. 2019;23:4711–22. https://doi.org/10.1111/jcmm.14394.
Li S, Huang H, Xing M, Qin J, Zhang H, Liu Y, et al. Carbon Ion induces cell death and G2/M arrest through pRb/E2F1Chk2/Cdc2 signaling pathway in X-ray resistant B16F10 melanoma cells. Dose Response. 2022;20:15593258221092364. https://doi.org/10.1177/15593258221092364.
Charalampopoulou A, Barcellini A, Frittitta GE, Fulgini G, Ivaldi GB, Magro G et al. In Vitro effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours. Life 2023;13. https://doi.org/10.3390/life13030794.
Charalampopoulou A, Barcellini A, Carnevale F, Ciocca M, Faris P, Moccia F, et al. PD-0489 effect of C-ions on activation of mucosal melanoma cells through alterations in Ca2 + signaling. Radiother Oncol. 2022;170:S439. https://doi.org/10.1016/S0167-8140(22)02860-2.
Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. https://doi.org/10.3389/fonc.2012.00095.
Yoshimoto Y, Oike T, Okonogi N, Suzuki Y, Ando K, Sato H, et al. Carbon-ion beams induce production of an immune mediator protein, high mobility group box 1, at levels comparable with X-ray irradiation. J Radiat Res. 2015;56:509–14. https://doi.org/10.1093/jrr/rrv007.
Onishi M, Okonogi N, Oike T, Yoshimoto Y, Sato H, Suzuki Y, et al. High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J Radiat Res. 2018;59:541–6. https://doi.org/10.1093/jrr/rry049.
Iijima M, Okonogi N, Nakajima NI, Morokoshi Y, Kanda H, Yamada T, et al. Significance of PD-L1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J Gynecol Oncol. 2020;31:e19. https://doi.org/10.3802/jgo.2020.31.e19.
Zhou H, Tu C, Yang P, Li J, Kepp O, Li H, et al. Carbon ion radiotherapy triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy in mice. Oncoimmunology. 2022;11:2057892. https://doi.org/10.1080/2162402X.2022.2057892.
Wakatsuki M, Kato S, Ohno T, Kiyohara H, Karasawa K, Tamaki T, et al. Difference in distant failure site between locally advanced squamous cell carcinoma and adenocarcinoma of the uterine cervix after C-ion RT. J Radiat Res. 2015;56:523–8. https://doi.org/10.1093/jrr/rru117.
Okonogi N, Wakatsuki M, Kato S, Karasawa K, Kiyohara H, Shiba S, et al. Clinical outcomes of carbon ion radiotherapy with concurrent chemotherapy for locally advanced uterine cervical adenocarcinoma in a phase 1/2 clinical trial (protocol 1001). Cancer Med. 2018;7:351–9. https://doi.org/10.1002/cam4.1305.
Wakatsuki M, Kato S, Kiyohara H, Ohno T, Karasawa K, Tamaki T, et al. Clinical trial of prophylactic extended-field carbon-ion radiotherapy for locally advanced uterine cervical cancer (protocol 0508). PLoS ONE. 2015;10:e0127587. https://doi.org/10.1371/journal.pone.0127587.
Okonogi N, Wakatsuki M, Kato S, Murata H, Kiyohara H, Karasawa K, et al. Significance of concurrent use of weekly cisplatin in carbon-ion radiotherapy for locally advanced adenocarcinoma of the uterine cervix: a propensity score-matched analysis. Cancer Med. 2020;9:1400–8. https://doi.org/10.1002/cam4.2784.
Zhang J, Qin L, Chen H-M, Hsu H-C, Chuang C-C, Chen D, et al. Overall survival, locoregional recurrence, and distant metastasis of definitive concurrent chemoradiotherapy for cervical squamous cell carcinoma and adenocarcinoma: before and after propensity score matching analysis of a cohort study. Am J Cancer Res. 2020;10:1808–20.
Miyasaka Y, Yoshimoto Y, Murata K, Noda S-E, Ando K, Ebara T, et al. Treatment outcomes of patients with adenocarcinoma of the uterine cervix after definitive radiotherapy and the prognostic impact of tumor-infiltrating CD8 + lymphocytes in pre-treatment biopsy specimens: a multi-institutional retrospective study. J Radiat Res. 2020;61:275–84. https://doi.org/10.1093/jrr/rrz106.
Farley JH, Hickey KW, Carlson JW, Rose GS, Kost ER, Harrison TA. Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer. 2003;97:2196–202. https://doi.org/10.1002/cncr.11371.
Niibe Y, Kenjo M, Onishi H, Ogawa Y, Kazumoto T, Ogino I, et al. High-dose-rate intracavitary brachytherapy combined with external beam radiotherapy for stage IIIb adenocarcinoma of the uterine cervix in Japan: a multi-institutional study of Japanese Society of Therapeutic Radiology and Oncology 2006–2007 (study of JA. Jpn J Clin Oncol. 2010;40:795–9. https://doi.org/10.1093/jjco/hyq053.
Okonogi N, Ando K, Murata K, Wakatsuki M, Noda S-E, Irie D, et al. Multi-institutional retrospective analysis of Carbon-Ion Radiotherapy for patients with locally Advanced Adenocarcinoma of the Uterine Cervix. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13112713.
Ohno T, Noda S-E, Murata K, Yoshimoto Y, Okonogi N, Ando K, et al. Phase I study of Carbon Ion Radiotherapy and Image-guided brachytherapy for locally Advanced Cervical Cancer. Cancers (Basel). 2018;10. https://doi.org/10.3390/cancers10090338.
Okonogi N, Murata K, Yamada S, Habu Y, Hori M, Kurokawa T, et al. A phase ib study of Durvalumab (MEDI4736) in combination with Carbon-Ion Radiotherapy and Weekly Cisplatin for patients with locally Advanced Cervical Cancer (DECISION Study): the early safety and efficacy results. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms241310565.
Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Melanoma of the lower genital tract: prognostic factors and treatment modalities. Gynecol Oncol. 2018;150:180–9. https://doi.org/10.1016/j.ygyno.2018.04.562.
Cuccia F, D’Alessandro S, Blasi L, Chiantera V, Ferrera G. The role of Radiotherapy in the management of vaginal melanoma: a Literature Review with a focus on the potential synergistic role of Immunotherapy. J Pers Med. 2023;13. https://doi.org/10.3390/jpm13071142.
Murata H, Okonogi N, Wakatsuki M, Kato S, Kiyohara H, Karasawa K, et al. Long-term outcomes of Carbon-Ion Radiotherapy for Malignant Gynecological Melanoma. Cancers (Basel). 2019;11:482. https://doi.org/10.3390/cancers11040482.
Cavalieri S, Ronchi S, Barcellini A, Bonora M, Vischioni B, Vitolo V, et al. Toxicity of carbon ion radiotherapy and immune checkpoint inhibitors in advanced melanoma. Radiother Oncol. 2021. https://doi.org/10.1016/J.RADONC.2021.08.021.
Barcellini A, Vitolo V, Lazzari R, Consoli F, Ditto A, Facoetti A, et al. P178 Carbon-Ion radiotherapy for malignant gynecological melanoma. Int J Gynecol Cancer. 2019;29. https://doi.org/10.1136/ijgc-2019-ESGO.238. :A165 LP-A166.
Barcellini A, Vitolo V, Facoetti A, Fossati P, Preda L, Fiore MR, et al. Feasibility of carbon ion radiotherapy in the treatment of gynecological melanoma. Vivo (Brooklyn). 2019;33. https://doi.org/10.21873/invivo.11497.
Barcellini A, Roccio M, Laliscia C, Zanellini F, Pettinato D, Valvo F, et al. Endometrial Cancer: when upfront surgery is not an option. Oncol. 2020. https://doi.org/10.1159/000510690.
Irie D, Okonogi N, Wakatsuki M, Kato S, Ohno T, Karasawa K, et al. Carbon-ion radiotherapy for inoperable endometrial carcinoma. J Radiat Res. 2018;59:309–15. https://doi.org/10.1093/jrr/rry003.
Draghini L, Maranzano E, Casale M, Trippa F, Anselmo P, Arcidiacono F, et al. Definitive three-dimensional high-dose-rate brachytherapy for inoperable endometrial cancer. J Contemp Brachytherapy. 2017;9:118–23. https://doi.org/10.5114/jcb.2017.67454.
Gill BS, Kim H, Houser C, Olsen A, Kelley J, Edwards RP, et al. Image-based three-dimensional conformal brachytherapy for medically inoperable endometrial carcinoma. Brachytherapy. 2014;13:542–7. https://doi.org/10.1016/j.brachy.2014.07.002.
Acharya S, Perkins SM, DeWees T, Fischer-Valuck BW, Mutch DG, Powell MA, et al. Brachytherapy is Associated with Improved Survival in Inoperable Stage I endometrial adenocarcinoma: a Population-based analysis. Int J Radiat Oncol Biol Phys. 2015;93:649–57. https://doi.org/10.1016/j.ijrobp.2015.06.013.
Barcellini A, Gadducci A, Laliscia C, Imparato S, Vitolo V, Preda L et al. Adenoid cystic carcinoma of Bartholin’s gland. What Is Best Approach? Oncol 2020:1–7. https://doi.org/10.1159/000506485.
Loap P, Vischioni B, Bonora M, Ingargiola R, Ronchi S, Vitolo V, et al. Biological Rationale and Clinical Evidence of Carbon Ion Radiation Therapy for Adenoid Cystic Carcinoma: a narrative review. Front Oncol. 2021;11:789079. https://doi.org/10.3389/fonc.2021.789079.
Bernhardt D, Sterzing F, Adeberg S, Herfarth K, Katayama S, Foerster R, et al. Bimodality treatment of patients with pelvic adenoid cystic carcinoma with photon intensity-modulated radiotherapy plus carbon ion boost: a case series. Cancer Manag Res. 2018;10:583–8. https://doi.org/10.2147/CMAR.S148395.
Shiba S, Okonogi N, Kato S, Wakatsuki M, Kobayashi D, Kiyohara H, et al. Clinical impact of re-irradiation with Carbon-ion Radiotherapy for Lymph Node Recurrence of Gynecological Cancers. Anticancer Res. 2017;37:5577–83. https://doi.org/10.21873/anticanres.11991.
Critelli P, Pezzulla D, Lillo S, Arpa D, Scricciolo M, Di Carlo C, et al. Outcomes and toxicity in re-irradiation of gynecologic cancer: systematic review of the Italian association of radiation and clinical oncology (AIRO). Gynecol Oncol. 2023;179:33–41. https://doi.org/10.1016/j.ygyno.2023.10.016.
Barcellini A, Vitolo V, Lazzari R, Cobianchi L, Biffi R, Facoetti A, et al. EP696 inoperable pelvic sidewall recurrence of gynecological cancer treated with proton and carbon ion radiotherapy: CNAO preliminary experience. Int J Gynecol Cancer. 2019;29. https://doi.org/10.1136/ijgc-2019-ESGO.750. A397 LP-A397.
Cuccia F, Pastorello E, Vitale C, Nicosia L, Mazzola R, Figlia V, et al. The use of SBRT in the management of oligometastatic gynecological cancer: report of promising results in terms of tolerability and clinical outcomes. J Cancer Res Clin Oncol. 2021;147:3613–8. https://doi.org/10.1007/s00432-021-03802-4.
Macchia G, Lazzari R, Colombo N, Laliscia C, Capelli G, D’Agostino GR, et al. A large, Multicenter, Retrospective Study on Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Ovarian Cancer (MITO RT1 Study): a collaboration of MITO, AIRO GYN, and MaNGO groups. Oncologist. 2020;25:e311–20. https://doi.org/10.1634/theoncologist.2019-0309.
Macchia G, Pezzulla D, Campitelli M, Laliscia C, Fodor A, Bonome P, et al. Efficacy and safety of stereotactic body Radiation Therapy in Oligometastatic Uterine Cancer (MITO-RT2/RAD): a large, real-world study in Collaboration with Italian Association of Radiation Oncology, Multicenter Italian trials in Ovarian Cancer, and Mari. Int J Radiat Oncol Biol Phys. 2023;117:321–32. https://doi.org/10.1016/j.ijrobp.2023.04.025.
Barcellini A, Charalampopoulou A, De Cecco L, Fodor A, Rabaiotti E, Candotti G, et al. Ovarian Cancer radiosensitivity: what have we understood so Far? Life (Basel Switzerland). 2022;13. https://doi.org/10.3390/life13010006.
Pereira S, Orlandi E, Deneuve S, Barcellini A, Chalaszczyk A, Behm-Ansmant I, et al. The normal, the Radiosensitive, and the ataxic in the era of Precision Radiotherapy: a narrative review. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14246252.
Sokol O, Durante M. Carbon ions for hypoxic tumors: are we making the most of them? Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15184494.
Bi Y, Verginadis II, Dey S, Lin L, Guo L, Zheng Y, et al. Radiosensitization by the PARP inhibitor olaparib in BRCA1-proficient and deficient high-grade serous ovarian carcinomas. Gynecol Oncol. 2018;150:534–44. https://doi.org/10.1016/j.ygyno.2018.07.002.
Gerelchuluun A, Manabe E, Ishikawa T, Sun L, Itoh K, Sakae T, et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res. 2015;183:345–56. https://doi.org/10.1667/RR13904.1.
Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA, et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;116:374–80. https://doi.org/10.1016/j.radonc.2015.08.014.
Aibe N, Ogino H, Wakatsuki M, Fujikawa K, Teramukai S, Fukumitsu N, et al. Comprehensive analysis of Japanese nationwide cohort data of particle beam therapy for pulmonary, liver and lymph node oligometastases: particle beam therapy versus high-precision X-ray radiotherapy. J Radiat Res. 2023;64:i69–83. https://doi.org/10.1093/jrr/rrad004.
Barcellini A, Murata K, Fontana G, Ghirelli A, Vai A, Molinelli S, et al. PD-0810 pilot study on carbon-ion radiotherapy for recurrent/refractory ovarian/salpinx cancer. Radiother Oncol. 2023;182:S682–3. https://doi.org/10.1016/S0167-8140(23)08992-2.
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. https://doi.org/10.1093/jnci/djs629.
Durante M, Formenti S. Harnessing radiation to improve immunotherapy: better with particles? Br J Radiol. 2020;93:20190224. https://doi.org/10.1259/bjr.20190224.
Boustani J, Grapin M, Laurent P-A, Apetoh L, Mirjolet C. The 6th R of Radiobiology: reactivation of Anti-tumor Immune Response. Cancers. 2019;11. https://doi.org/10.3390/cancers11060860.
Golden EB, Formenti SC. Is tumor (R)ejection by the immune system the 5th R. Radiobiology? Oncoimmunology. 2014;3:e28133. https://doi.org/10.4161/onci.28133.
Pavone M, Autorino R, Bizzarri N, Chilorio G, Valentini V, Corrado G, et al. Uterine transposition versus uterine ventrofixation before radiotherapy as a fertility sparing option in young women with pelvic malignancies: systematic review of the literature and dose simulation. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2023;50:107270. https://doi.org/10.1016/j.ejso.2023.107270.
Barcellini A, Cassani C, Orlandi E, Nappi RE, Broglia F, Delmonte MP et al. Is motherhood still possible after pelvic carbon ion radiotherapy? A promising combined fertility-preservation approach. Tumori 2024:3008916231218794. https://doi.org/10.1177/03008916231218794.
Images. Created with BioRender.com n.d.
Funding
The authors declare that they have no funding and no financial support.
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors had full access and verified the underlying data reported in the manuscript and accept responsibility to submit for publication. Concept and design: Dr Barcellini; Methodology: Dr Barcellini; Acquisition and interpretation of data: Dr Barcellini and Dr Charalampopoulou; Drafting of the manuscript: Dr Barcellini; Supervision: Prof Locati and Prof Orlandi; Critical revision of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Ethical statement
Not applicable.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
Authors declare no conflict of interest with regard to the current article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barcellini, A., Charalampopoulou, A., Franzetti, J. et al. Carbon ion radiotherapy in gynaecological oncology: where we are and where we are headed. Health Technol. 14, 859–866 (2024). https://doi.org/10.1007/s12553-024-00863-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-024-00863-6