Abstract
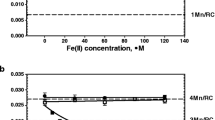
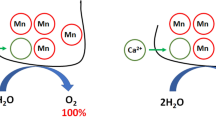
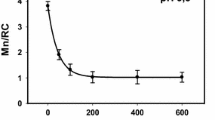
Water oxidation in photosystem II (PSII) is performed by the oxygen-evolving complex Mn4CaO5 which can be extracted from PSII and then reconstructed using exogenous cations Mn(II) and Ca2+. The binding efficiency of other cations to the Mn-binding sites in Mn-depleted PSII was investigated without any positive results. At the same time, a study of the Fe cations interaction with Mn-binding sites showed that it binds at a level comparable with the binding of Mn cations. Binding of Fe(II) cations first requires its light-dependent oxidation. In general, the interaction of Fe(II) with Mn-depleted PSII has a number of features similar to the two-quantum model of photoactivation of the complex with the release of oxygen. Interestingly, incubation of Ca-depleted PSII with Fe(II) cations under certain conditions is accompanied by the formation of a chimeric cluster Mn/Fe in the oxygen-evolving complex. PSII with the cluster 2Mn2Fe was found to be capable of water oxidation, but only to the H2O2 intermediate. However, the cluster 3Mn1Fe can oxidize water to O2 with an efficiency about 25% of the original in the absence of extrinsic proteins PsbQ and PsbP. In the presence of these proteins, the efficiency of O2 evolution can reach 80% of the original when adding exogenous Ca2+. In this review, we summarized information on the formation of chimeric Mn–Fe clusters in the oxygen-evolving complex. The data cited may be useful for detailing the mechanism of water oxidation.


Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper including references.
Abbreviations
- Chl:
-
Chlorophyll
- D1:
-
Integral protein of the photosystem II reaction center
- DPC:
-
Diphenylcarbazide
- EP:
-
Extrinsic proteins
- HAS:
-
High-affinity Mn-binding site
- H2Q:
-
Hydroquinone
- LAS:
-
Low-affinity Mn-binding site
- OEC:
-
Oxygen-evolving complex
- PSII:
-
Photosystem II
- PSII(-Ca) :
-
Ca2+-depleted PSII membranes
- PSII(-Mn) :
-
Mn-depleted PSII membranes
- RC:
-
Reaction center
- Tris:
-
Tris(hydroxymethyl)amino methane
- SOD:
-
Superoxide dismutase
- YZ :
-
Redox-active tyrosine D1-Tyr161, the first electron donor to P680+ in PSII
References
Atta M, Nordlund P, Aberg A, Eklund H, Fontecave M (1992) Substitution of manganese for iron in ribonucleotide reductase from Escherichia coli. Spectroscopic and crystallographic characterization. J Biol Chem 267(29):20682–20688. https://doi.org/10.1016/S0021-9258(19)36739-0
Avramov AP, Hwang HJ, Burnap RL (2020) The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc Natl Acad Sci USA 117:28036–28045. https://doi.org/10.1073/pnas.2011315117
Bernát G, Morvaridi F, Feyziyev Y, Styring S (2002) pH dependence of the four individual transitions in the catalytic S-cycle during photosynthetic oxygen evolution. Biochemistry 41:5830–5843. https://doi.org/10.1021/bi011691u
Charley PJ, Sarkar B, Stitt CF, Saltman P (1963) Chelation of iron by sugars. Biochim Biophys Acta 69:313–321. https://doi.org/10.1016/0006-3002(63)91264-2
Damoder R, Dismukes GC (1984) pH dependence of the multiline, manganese EPR signal for the ’S2’ state in PS II particles. Absence of proton release during the S1 → S2 electron transfer step of the oxygen evolving system. FEBS Lett 174:157–161. https://doi.org/10.1016/0014-5793(84)81096-0
Gates C, Ananyev G, Roy-Chowdhury S, Cullinane B, Miller M, Fromme P, Dismukes GC (2022) Why did nature choose manganese over cobalt to make oxygen photosynthetically on the earth? J Phys Chem B 126(17):3257–3268. https://doi.org/10.1021/acs.jpcb.2c00749
Ghanotakis DF, Babcock GT, Yocum CF (1984a) Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted photosystem II preparations. FEBS Lett 167:127–130. https://doi.org/10.1016/0014-5793(84)80846-7
Ghanotakis DF, Topper JN, Yocum CF (1984b) Exogenous reductants reduce and destroy the Mn-complex in photosystem II membranes depleted of the 17 and 23 kDa polypeptides. Biochim Biophys Acta 767:524–531. https://doi.org/10.1016/0005-2728(84)90051-3
Ghanotakis DF, Babcock GT, Yocum CF (1985) Structure of the oxygen-evolving complex of Photosystem II: calcium and lanthanum compete for sites on the oxidizing side of Photosystem II which control the binding of water-soluble polypeptides and regulate the activity of the manganese complex. Biochim Biophys Acta 809:173–180. https://doi.org/10.1016/0005-2728(85)90060-X
Ghirardi ML, Lutton TW, Seibert M (1996) Interactions between diphenylcarbazide, zinc, cobalt, and manganese on the oxidizing side of photosystem II. Biochemistry 35:1820–1828. https://doi.org/10.1021/bi951657d
Ghosh I, Khan S, Banerjee G, Dziarski A, Vinyard DJ, Debus RJ, Brudvig GW (2019) Insights into proton-transfer pathways during water oxidation in photosystem II. J Phys Chem B 123:8195–8202. https://doi.org/10.1021/acs.jpcb.9b06244
Gisriel CJ, Zhou K, Huang H-L, Debus RJ, Xiong Y, Brudvig GW (2020) Cryo-EM structure of monomeric photosystem II from Synechocystis sp. PCC 6803 lacking the water-oxidation complex. Joule 4(10):2131–2148. https://doi.org/10.1016/j.joule.2020.07.016
Haddy A, Hatchell JA, Kimel RA, Thomas R (1999) Azide as a competitor of chloride in oxygen evolution by photosystem II. Biochemistry 38:6104–6110. https://doi.org/10.1021/bi983075c
Hoganson CW, Ghanotakis DF, Babcock GT, Yocum CF (1989) Mn2+ reduces YZ+ in manganese-depleted Photosystem II preparations. Photosynth Res 22:285–293. https://doi.org/10.1007/BF00048306
Hsu BD, Lee J-Y, Pan R-L (1987) The high-affinity binding site for manganese on the oxidizing side of photosystem II. Biochim Biophys Acta 890(1):89–96. https://doi.org/10.1016/0005-2728(87)90072-7
Kálmán L, Flores M, Williams JC, Allen JP (2010) Electronic structure of Fe3+ at a metal-binding site introduced in modified bacterial reaction centers. Appl Magn Reson 37:27–37. https://doi.org/10.1007/s00723-009-0037-z
Kawakami K, Umena Y, Kamiya N, Shen J-R (2011) Structure of the catalytic, inorganic core of oxygen-evolving photosystem II at 1.9 Å resolution. J Photochem Photobiol B 104(1–2):9–18. https://doi.org/10.1016/j.jphotobiol.2011.03.017
Kern J, Chatterjee R, Young ID, Fuller FD, Lassalle L, Ibrahim M, Gul S, Fransson T, Brewster AS, Alonso-Mori R et al (2018) Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563:421–425. https://doi.org/10.1038/s41586-018-0681-2
Kondo M, Tatewaki H, Masaoka S (2021) Design of molecular water oxidation catalysts with earth-abundant metal ions. Chem Soc Rev 50(12):6790–6831. https://doi.org/10.1039/D0CS01442G
Kudasheva DS, Lai J, Ulman A, Cowman MK (2004) Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J Inorg Biochem 98:1757–1769. https://doi.org/10.1016/j.jinorgbio.2004.06.010
Kuntzleman T, McCarrick R, Penner-Hahn J, Yocum C (2004) Probing reactive sites within the photosystem II manganese cluster: evidence for separate populations of manganese that differ in redox potential. Phys Chem Chem Phys 6:4897–4904. https://doi.org/10.1039/B406601D
Kurashov VN, Lovyagina ER, Shkolnikov DYu, Solntsev MK, Mamedov MD, Semin BK (2009) Investigation of the low-affinity oxidation site for exogenous electron donors in the Mn-depleted photosystem II complexes. Biochim Biophys Acta 1787:1492–1498. https://doi.org/10.1016/j.bbabio.2009.07.002
Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML (1995) Structure-function in Escherichia coli iron superoxide dismutase: comparisons with the manganese enzyme from Thermus thermophilus. Biochemistry 34(5):1646–1660. https://doi.org/10.1021/bi00005a021
Lovyagina ER, Davletshina LN, Kultysheva MYu, Timofeev KN, Ivanov II, Semin BK (2005) Characteristic features of the interaction between Fe(II) cations and the donor side of the manganese-depleted photosystem II. Russ J Plant Physiol 52(1):7–14. https://doi.org/10.1007/s11183-005-0002-0
Lovyagina ER, Loktyushkin AV, Semin BK (2021) Effective binding of Tb3+ and La3+ cations on the donor side of Mn-depleted photosystem II. J Biol Inorg Chem 26:1–11. https://doi.org/10.1007/s00775-020-01832-w
Lubitz W, Chrysina M, Cox N (2019) Water oxidation in photosystem II. Photosynth Res 142(1):105–125. https://doi.org/10.1007/s11120-019-00648-3
Najafpour M, Heidari S, Balaghi SE, Hołyńska M, Sadr MH, Soltani B, Khatamian M, Larkum AW, Allakhverdiev SI (2017) Proposed mechanisms for water oxidation by Photosystem II and nanosized manganese oxides. Biochim Biophys Acta 2:156–174. https://doi.org/10.1016/j.bbabio.2016.11.007
Oliver N, Avramov AP, Nürnberg DJ, Dau H, Burnap RL (2022) From manganese oxidation to water oxidation: assembly and evolution of the water-splitting complex in photosystem II. Photosynth Res 152(2):107–133. https://doi.org/10.1007/s11120-022-00912-z
Ono T (2000) Effects of lanthanide substitution at Ca2+-site on the properties of the oxygen evolving center of photosystem II. J Inorg Biochem 82:85–91. https://doi.org/10.1016/S0162-0134(00)00144-6
Ono T, Inoue Y (1988) Discrete extraction of the Ca atom functional for O2 evolution in higher plant photosystem II by a simple low pH treatment. FEBS Lett 227:147–152. https://doi.org/10.1016/0014-5793(88)80886-X
Ono T, Inoue Y (1990) Abnormal redox reactions in photosynthetic O2-evolving centers in NaCl/EDTA-washed PS II. A dark-stable EPR multiline signal and an unknown positive charge accumulator. Biochim Biophys Acta 1020:269–277. https://doi.org/10.1016/0005-2728(90)90157-Y
Pace RJ, Stranger R, Petrie S (2012) Why nature chose Mn for the water oxidase in photosystem II. Dalton Trans 41(24):7179–7189. https://doi.org/10.1039/C2DT30185G
Saito K, Shen J-R, Ishida T, Ishikita H (2011) Short hydrogen bond between redox-active tyrosine YZ and D1-His190 in the photosystem II crystal structure. Biochemistry 50:9836–9844. https://doi.org/10.1021/bi201366j
Saito K, Nakagawa M, Mandal M, Ishikita H (2021) Role of redox-inactive metals in controlling the redox potential of heterometallic manganese–oxido clusters. Photosynth Res 148:153–159. https://doi.org/10.1007/s11120-021-00846-y
Saito M, Saito K, Ishikita H (2023) Structural and energetic insights into Mn-to-Fe substitution in the oxygen-evolving complex. iScience 26(8):107352. https://doi.org/10.1016/j.isci.2023.107352
Schiller H, Dau H (2000) Preparation protocols for high-activity photosystem II membrane particles of green algae and higher plants, pH dependence of oxygen evolution and comparison of the S2-state multiline signal by X-band EPR spectroscopy. J Photochem Photobiol B 55:138–144. https://doi.org/10.1016/S1011-1344(00)00036-1
Schlodder E, Meyer B (1987) pH dependence of oxygen evolution and reduction kinetics of photooxidized chlorophyll aII (P-680) in Photosystem II particles from Synechococcus sp. Biochim Biophys Acta 890:23–31. https://doi.org/10.1016/0005-2728(87)90064-8
Semin BK, Davletshina LN (2023) High-efficiency oxygen evolution by photosystem II oxygen-evolving complex containing 3Mn per reaction center. J Biol Inorg Chem 28:393–401. https://doi.org/10.1007/s00775-023-01987-2
Semin BK, Davletshina LN, Rubin AB (2015) Correlation between pH dependence of O2 evolution and sensitivity of Mn cations in the oxygen-evolving complex to exogenous reductants. Photosynth Res 125:95−103. https://doi.org/10.1007/s11120-015-0155-4
Semin BK, Seibert M (2004) Iron bound to the high-affinity Mn-binding site of the oxygen-evolving complex shifts the pK of a component controlling electron transport via Y(Z). Biochemistry 43:6772–6782. https://doi.org/10.1021/bi036047p
Semin BK, Seibert M (2006a) A carboxylic residue at the high-affinity, Mn-binding site participates in the binding of iron cations that block the site. Biochim Biophys Acta 1757(3):189–197. https://doi.org/10.1016/j.bbabio.2006.02.001
Semin BK, Seibert M (2006b) Flash-induced blocking of the high-affinity manganese-binding site in photosystem II by iron cations: dependence on the dark interval between flashes and binary oscillations of fluorescence yield. J Phys Chem B 110:25532–25542. https://doi.org/10.1021/jp0652796
Semin BK, Seibert M (2016) Substituting Fe for two of the four Mn ions in photosystem II–effects on water-oxidation. J Bioenerg Biomembr 48:227–240. https://doi.org/10.1007/s10863-016-9651-2
Semin BK, Ivanov II, Rubin AB, Parak F (1995) High-specific binding of Fe(II) at the Mn-binding site in Mn-depleted PSII membranes from spinach. FEBS Lett 375:223–226. https://doi.org/10.1016/0014-5793(95)01215-Z
Semin BK, Ghirardi ML, Seibert M (2002) Blocking of electron donation by Mn(II) to YZ• following incubation of Mn-depleted photosystem II membranes with Fe(II) in the light. Biochemistry 41:5854–5864. https://doi.org/10.1021/bi0200054
Semin BK, Davletshina LN, Aleksandrov AYu, Lanchinskaya VYu, Novakova AA, Ivanov II (2004) pH dependence of iron binding to the donor side of photosystem II. Biochem Mosc 69:410–419. https://doi.org/10.1023/B:BIRY.0000022066.38297.8a
Semin BK, Lovyagina ER, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2005) Iron-blocking the high-affinity Mn-binding site in photosystem II facilitates identification of the type of hydrogen bond participating in proton-coupled electron transport via YZ. Biochemistry 44:9746–9757. https://doi.org/10.1021/bi047618w
Semin BK, Davletshina LN, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2013) Production of reactive oxygen species in decoupled, Ca2+-depleted PSII and their use in assigning a function to chloride on both sides of PSII. Photosynth Res 117(1):385–399. https://doi.org/10.1007/s11120-013-9870-x
Semin BK, Davletshina LN, Seibert M, Rubin AB (2018) Creation of a 3Mn/1Fe cluster in the oxygen-evolving complex of photosystem II and investigation of its functional activity. J Photochem Photobiol B 178:192–200. https://doi.org/10.1016/j.jphotobiol.2017.11.016
Semin BK, Davletshina LN, Goryachev SN, Seibert M (2021) Ca2+ effects on Fe(II) interactions with Mn-binding sites in Mn-depleted oxygen-evolving complexes of photosystem II and on Fe replacement of Mn in Mn-containing. Ca-Depleted Complexes Photosynth Res 147(2):229–237. https://doi.org/10.1007/s11120-020-00813-z
Shen JR (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48. https://doi.org/10.1146/annurev-arplant-050312-120129
Shen J-R, Inoue Y (1991) Low pH-induced dissociation of three extrinsic proteins from O2-evolving photosystem II. Plant Cell Physiol 32(3):453–457. https://doi.org/10.1093/oxfordjournals.pcp.a078101
Shevela D, Kern JF, Govindjee G, Messinger J (2023) Solar energy conversion by photosystem II: principles and structures. Photosynth Res 156:279–307. https://doi.org/10.1007/s11120-022-00991-y
Sillen LG, Marten AE (1964) Stability constants of metal–ion complexes, Special Publ 17. The Chemical Society, Burlington House, London, p W1
Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R (2015) Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517:99–103. https://doi.org/10.1038/nature13991
Suga M, Akita F, Sugahara M, Kubo M, Nakajima Y, Nakane T, Yamashita K, Umena Y, Nakabayashi M, Yamane T et al (2017) Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543:131–135. https://doi.org/10.1038/nature21400
Suga M, Akita F, Yamashita K, Nakajima Y, Ueno G, Li H, Yamane T, Hirata K, Umena Y, Yonekura S et al (2019) An oxyl/oxo mechanism for oxygen-oxygen coupling in PSII revealed by an X-ray free-electron laser. Science 366(6463):334–338. https://doi.org/10.1126/science.aax6998
Suzuki H, Sugiura M, Noguchi T (2005) pH dependence of the flash-Induced S-state transitions in the oxygen-evolving center of photosystem II from Thermosynechoccocus elongatus as revealed by Fourier transform infrared spectroscopy. Biochemistry 44:1708–1718. https://doi.org/10.1021/bi0483312
Tamura N, Cheniae GM (1987) Photoactivation of the water-oxidizing complex in Photosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical and kinetic characterization. Biochim Biophys Acta 890:179–194. https://doi.org/10.1016/0005-2728(87)90019-3
Tang K, Williams JC, Allen JP, Kálmán L (2009) Effect of anions on the binding and oxidation of divalent manganese and iron in modified bacterial reaction centers. Biophys J 96(8):3295–3304. https://doi.org/10.1016/j.bpj.2009.01.027
Tsui EY, Agapie T (2013) Reduction potentials of heterometallic manganese–oxido cubane complexes modulated by redox-inactive metals. Proc Natl Acad Sci USA 110:10084–10088. https://doi.org/10.1073/pnas.1302677110
Tsui EY, Tran R, Yano J, Agapie T (2013) Redox-inactive metals modulate the reduction potential in heterometallic manganese-oxido clusters. Nat Chem 5(4):293–299. https://doi.org/10.1038/nchem.1578
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60. https://doi.org/10.1038/nature09913
Vander Meulen KA, Hobson A, Yocum CF (2002) Calcium depletion modifies the structure of the photosystem II O2-evolving complex. Biochemistry 41:958–966. https://doi.org/10.1021/bi0109414
Vander Meulen KA, Hobson A, Yocum CF (2004) Reconstitution of the photosystem II Ca2+ binding site. Biochim Biophys Acta 1655:179–183. https://doi.org/10.1016/j.bbabio.2003.08.012
Vass I, Styring S (1991) pH-Dependent charge equilibria between tyrosine-D and the S states in photosystem II. Estimation of relative midpoint redox potentials. Biochemistry 30:830–839. https://doi.org/10.1021/bi00217a037
Vrettos JS, Stone DA, Brudvig GW (2001) Quantifying the ion selectivity of the Ca2+ site in photosystem II. Evidence for direct involvement of Ca2+ in O2 formation. Biochemistry 40:7937–7945. https://doi.org/10.1021/bi010679z
Waggoner CM, Yocum CF (1990) Calcium activated oxygen evolution. In: Baltscheffsky M (ed) Current research in photosynthesis, Springer, Dordrecht, pp 733–736. https://doi.org/10.1007/978-94-009-0511-5_167
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362(6423):834–836. https://doi.org/10.1038/362834a0
Zhang M, Bommer M, Chatterjee R, Hussein R, Yano J, Dau H, Kern J, Dobbek H, Zouni A (2017) Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 6:e26933. https://doi.org/10.7554/eLife.26933
Funding
The research was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University No. 121032500058–7.
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Semin, B., Loktyushkin, A. & Lovyagina, E. Current analysis of cations substitution in the oxygen-evolving complex of photosystem II. Biophys Rev 16, 237–247 (2024). https://doi.org/10.1007/s12551-024-01186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-024-01186-6