Abstract
Amorphous protein aggregates are oligomers that lack specific, high-order structures. Soluble amorphous aggregates are smaller than ~1 µm. Despite their lack of high-order structure, amorphous protein aggregates exhibit specific biophysical properties such as reversibility of formation, density, conformation, and biochemical stability. Our mutational analysis using a Solubility Controlling Peptide (SCP) tag strongly suggests that amorphous aggregation of small globular proteins can significantly increase in vivo immune response and that the magnitude of enhanced immune responses depends on the aggregates’ biophysical and biochemical properties. We propose that SCP tags might help develop subunit (protein) adjuvant-free (immunostimulant-free) vaccines by controlling the aggregation propensity of target proteins.
Similar content being viewed by others
Amorphous aggregates, which do not form specific, high-order structures, have attracted little attention. Indeed, amorphous aggregates are often regarded as a technical problem that must be overcome to produce and use proteins effectively. The lack of interest was partially due to the idea that amorphous aggregation was not thought to be significantly associated with disease, a notable exception being cataracts (Boatz et al. 2017). However, reports of the loss of physiological functions related to intracellular amorphous aggregation and liquid–liquid phase separation (LLPS) have recently appeared, generating renewed interest in their biophysical characterization (Mitrea and Kriwacki 2016).
This review describes how the biophysical properties of amorphous protein aggregates can be characterized despite their lack of high-order structure. In addition, we briefly describe our mutational analysis of BPTI variants and other small globular proteins, suggesting a possible relationship between the biophysical/biochemical properties of amorphous aggregates and the increased in vivo immune response.
Mutational analysis of protein solubility
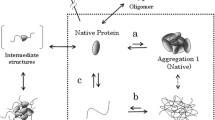
Until recently, amorphous protein aggregates have seldom been the subject of detailed, systematic, biophysical study (Hirota et al. 2019). On the other hand, protein solubility, which in many cases can be viewed as the counterpart of protein aggregation, has been thoroughly investigated from both physicochemical and biophysical viewpoints (Fig. 1).
Protein solubility is traditionally discussed in terms of hydrophobicity (Tanford 1997) or hydropathy (Kyte and Doolittle 1982). The hydrophilic/hydrophobic model was initially developed as an index calculated from the ratio of amino acids present in nonpolar and aqueous solutions (Nozaki and Tanford 1971), representing the inside and outside of proteins (Baldwin and Rose 2016). Thus, hydrophobicity/hydrophilicity is not as suitable as one might think for describing protein solubility.
Physicochemical studies of protein solubility include studies of cosolvent effects on protein solubility and structural stability, reflecting the concept of preferential solvation, as proposed by Timasheff’s group in the 1970s to 1990s (For reviews: Timasheff 2002; Arakawa 2018). Preferential solvation provides a molecular-level view of the salting-in/out effect, enabling a thermodynamic description of the influence of cosolvents on protein solubility and stability and is impacting several fields of biotechnology (Arakawa and Gagnon 2018).
Recently, Trevino et al. comprehensively replaced specific surface residues of RNase Sa with 20 different amino acids and examined changes in solubility (Trevino et al. 2007). This study is among the few examples of systematic mutational analysis to explore protein solubility from a physicochemical perspective. At about the same time, we reported how to substantially increase the solubility of a low-solubility BPTI variant (bovine pancreatic trypsin inhibitor protein; molecular weight 6.5 kDa; Fig. 2), BPTI-19A, by merely adding five Lys to the C-terminus of the protein (Kato et al. 2007; BPTI-19A (or -19A for short) is a BPTI variant with 19 alanine produced initially to assess the folding of BPTI; Kuroda and Kim 2000). Subsequent studies with five Arg, which have a positive charge like Lys, showed a similar effect, suggesting that the increased solubility is due to intermolecular electrostatic repulsion. This technique is a convenient way to increase the solubility of a protein without changing solvent conditions because the few charged amino acids added to protein termini usually do not disturb the structure and activity of the protein. As this technique was extended to other amino acid types (Islam et al. 2012) and various proteins (Rathnayaka et al. 2011; Nautiyal and Kuroda 2018; Brindha et al. 2021; Kibria et al. 2021; Brindha and Kuroda 2022), the attached peptides were named solubility-enhancing peptide tags (SEPs).
Hydrodynamic radii of BPTI-19A and SCP-tagged BPTI-19As. At the top is a schematic of BPTI-19A and BPTI-19A with an SCP tag consisting of 5 residues. Hydrodynamic radii were measured using DLS, and C5X stands for an SCP tag with 5 amino acids (X) attached at the C terminus of BPTI-19A (BPTI-C5I) with two Glycines as a linker. DLS conditions are as follows: protein concentration 1 mg/mL; pH 8.2; Tris buffer, 25 °C. Experimental details are described in Kabir et al. (2018)
We carried out a systematic analysis of the effect of adding short peptides to protein termini by measuring the solubility of BPTI-19A SEP tagged variants for 15 types of amino acids (all amino acids, but Cys, Trp, Tyr, Phe, and Thr) at pH 4.7 and pH 7.7 (Islam et al. 2012; Khan et al. 2013). We defined solubility as the amount of BTPI mutant remaining in the supernatant fraction after centrifugation of the protein sample (Islam et al. 2012). The advantage of this definition is that it is close to the generally accepted concept of solubility as being the amount of protein in the dissolved fraction of saturated samples. In addition, these experiments are performed at relatively low protein concentrations, which keeps sample viscosity low, ensuring reproducibility and accurate measurements, and permits analysis of aggregation speed or kinetics (Khan et al. 2013).
The pH dependency of amino acid solubility was consistent with trends expected from the charge status of side chains (Islam et al. 2012; Khan et al. 2013). That is, the solubility of Arg and Lys, which are positively charged at pHs 4.7–7.7, was equally high at both pHs, while the solubility of Glu and Asp, whose pKas are, respectively, 3.9 and 4.2, was high at neutral pH, but low at acidic pH. In addition, the solubility of His-tagged BPTI-19A was high at acidic pH but low at neutral pH. The pKa of His is 6.8, which explains why solubility is high at acidic pH but low at neutral pH. In the original hydrophobic/hydrophilic model, pH dependency was not considered, as all parameters were measured at neutral pHs. Determining hydrophobicity parameters at two or more pHs could integrate the pH dependency of amino acid solubility into the hydrophobicity/hydrophilicity model.
Biophysical properties of amorphous protein aggregates
Unlike amyloids and crystals, amorphous aggregates are irregularly shaped masses that do not form higher-ordered structures. Methods for analyzing the structure and biophysical properties of amorphous aggregates remain to be firmly established and despite a few exceptions, little attention has been paid to their biophysical properties other than their sizes (Iwura et al. 2014; Uchiyama et al. 2018; Ripple and Dimitrova 2012). However, amorphous aggregates have specific biophysical properties. For example, the biophysical properties of protein aggregates differ when the protein precipitates due to pH titration as opposed to heating. In the former case, aggregation is usually reversible, and the protein dissolves when the pH moves away from its isoelectric point. On the other hand, protein aggregation by thermal denaturation is usually irreversible, and the protein remains in the precipitate even when the sample temperature is lowered.
Although it is understood that amorphous aggregates might possess specific physicochemical characteristics other than size, few systematic studies have been conducted. To date, Nahri et al. proposed classifying aggregates based on five characteristics: size, reversibility/dissociation, conformation, chemical modification, and morphology (Narhi et al. 2012).
Biophysical analysis of BPTI aggregates produced using SCP tags
We performed a physicochemical analysis of aggregates formed by BPTI-19A with the SEP tag, originally designed to control a protein solubility (Kato et al. 2007). In our study, we used BPTI mutants with a SEP tag consisting of Arg, Lys, Leu, Ile, Val, Ala, Ser, Asp, Asn, Gln, Glu, Pro, and His. Henceforth, we refer to the SEP tag as a solubility controlling peptide (SCP) tag because some tags decreased the solubility of the protein. In this experiment, the aggregation states of BPTI mutants remaining in the supernatant after centrifugation were measured using dynamic light scattering (DLS) and static light scattering (SLS). The results showed that mutants with SCP tags consisting of five Ile (C5I) and Leu (C5L) formed aggregates of 3.5–4 nm and approximately 2 nm, respectively (Fig. 2). BPTI-19A with SCP tags composed of other amino acids did not form aggregates, according to DLS measurements, and their particle radii were similar to that of untagged BPTI-19A (~ 1.3 nm). We estimate that oligomers formed by BPTI-19A tagged with five Leu and Ile contain ~ 4 and 10–30 proteins, respectively (This is a rough estimate based on the volumes calculated using the hydrodynamic radii in Fig. 2).
To further characterize these aggregates, we analyzed the reversibility and temperature dependence of the aggregation process of BPTI-C5I. BPTI-C5I did not aggregate at 15 °C but reversibly formed 5-nm aggregates at 25 °C, according to DLS measurements (Fig. 3). These data show that aggregation of BPTI-C5I occurs within tens of seconds and that aggregate size remains constant for several minutes or probably longer. The reversible formation of oligomers (or small aggregates) with increasing temperature is explained by the fact that hydrophobicity increases as temperature increases.
Reversibility of aggregation of BPTI-19A (top) and BPTI-C5I; (BPTI-19A with 5 isoleucines attached to its C-terminus; center). The hydrodynamic radius (Rh) was measured using DLS. The incubation temperature is indicated by arrows below the axis. The experiment was carried out continuously, and time labeling on the horizontal axis is set to zero at each temperature change. BPTI-C5I was monomeric at 15 °C and aggregated when the temperature increased (center). This aggregation was reversible, and the particle radius decreased when the temperature was lowered to 15 °C (last 36 min). BPTI-C5I, which formed 5-nm aggregates at 37 °C, was unfolded according to circular dichroism (bottom panel), suggesting that BPTI-C5I oligomerizes in an unfolded state. In contrast, BPTI-19A was monomeric (top) and folded at temperatures between 15 and 37 °C. Experimental details are described in Kabir et al. (2018)
The properties of the amorphous aggregates were further characterized by biophysical methods (Kabir et al. 2018). Using circular dichroism and fluorescence spectroscopy, we assessed whether the three-dimensional structure of oligomerized BPTI is preserved in the aggregated state. Both circular dichroism and fluorescence indicated that the thermal stability of both BPTI-C5I and BPTI-C5L was reduced. In addition, a comparison with DLS showed that the two variants aggregate in a denatured or partially denatured state. We hypothesized that BPTI is denatured in the aggregates because BPTI, like other globular protein, is stabilized by the arrangement of hydrophobic residues being buried into the protein interior and hydrophilic residues being on its surface. The environment inside the aggregates is hydrophobic because of the Ile tag; thus, the difference in hydrophobicity between the interior of BPTI-C5I and its surface disappears, destabilizing BTPI-C5I (reverse hydrophobicity effect). The reverse hydrophobicity effect hypothesis was confirmed by detailed analysis using differential scanning calorimetry (Nakamura et al. 2012). The denaturation of BPTI-C5I was eventually summarized as an N <—> In <—> D reaction, in which the intermediate state is n-mer. We called this state an RO state (Reversible Oligomer state; Nakamura et al. 2012). The RO state has now been observed in native proteins and protein domains (Saotome et al. 2016, 2020; Onchaiya et al. 2022), suggesting that the RO state is not just an artifact resulting from the addition of an artificial SCP tag.
Finally, we assessed the biochemical stability of BPTI aggregates by limited proteolysis using pepsin (Kabir et al. 2018). The C5I and C5L mutants that aggregated at 37 °C were the most stable and did not degrade after 30 min of digestion. Conversely, variants that did not aggregate were rapidly digested. The increased biochemical stability of BPTI-C5I, which is maintained despite its reduced thermal stability, could be rationalized by hypothesizing that the molecular association of BPTI-C5I increased its resistance to protease digestion (Kabir et al. 2018).
Aggregation and immunogenicity
Aggregation has long been suspected to enhance protein immunogenicity (Gamble 1966). In particular, an increasing number of reports suggest that small, subvisible aggregates in protein preparations may trigger a surge in immune response (Ratanji et al. 2014; 2016; Roberts 2014; Marszal and Fowler 2012). Consequently, the U.S. Food and Drug Administration (FDA) issued a guideline in 2014 for assessing subvisible aggregates of 0.1–10 μm as quality control of recombinant protein products (Food and Drug Administration 2014).
We measured the immunogenicity of BPTI-19A and BPTI-C5I and three variants tagged with hydrophobic tags (Ala, Val, Leu, and Ile). Proteins were injected subcutaneously into mice, and IgG titers against BPTI-19A were measured using ELISA. As a result, we observed a clear correlation between immunogenicity and the oligomer radii (Fig. 4). In particular, BPTI-C5I induced a strong immune response with long-term immune memory that remained strong even 20 weeks after the last injection (booster injection).
IgG titer and particle radius measured using DLS under injection conditions. C5A, C5V, C5L, and C5I stand for BPTI-19A, with the respective amino acids attached to the C-terminus. 19A stands for BPTI-19A. N5I is a BPTI-19A with 5 isoleucines attached to the N-terminus. Serum IgG titers were measured with ELISA. 4-week-old JCl mice were injected subcutaneously with 30 µg of BPTI four times. Particle radii were measured with DLS just before injection by taking an aliquot from the injected sample; experimental details are described in Rahman et al. (2020a, b)
Finally, we studied the immunogenicity enhancement of the third domain of the dengue envelope protein (MW, 11 kDa, referred to hereafter as ED3; Fig. 5). ED3 possesses a site that is essential for membrane fusion between the virus and the host cell and an antibody recognition site, which are shared by flaviviruses (Dengue, Japanese encephalitis, Zika). As a result, ED3 is critical to viral infection, and anti-ED3 antisera have neutralizing ability against dengue virus (Crill and Roehrig 2001) and could serve as an antigen for vaccine development. However, the immune response against wild type ED3 is not strong enough for a vaccine. We are thus currently trying to enhance the immunogenicity of recombinant ED3 expressed in E. coli with the aim of producing a vaccine candidate (Rahman et al. 2021) that does not require the assistance of an adjuvant or immunostimulant. ED3 with increased immunogenicity might serve as a basis for subunit-vaccine development.
Immunogenic enhancement of dengue virus ED3 using a solubility-controlling peptide tag (SCP tag) (patent application 2017–152123). C4I stands for an ED3 with 4 isoleucines added to the C-terminus of ED3. Cntrl is antisera from mice injected with phosphate buffered saline (PBS) only. Titers were determined using ELISA plates coated with ED3 without an SCP tag. Crosses indicate the titers of individual mice. ED3 without a tag was monomeric. C4I formed oligomers of approximately 100 nm (Islam et al. 2020)
Still, successful development of ED3 variants that could serve as a vaccine remains challenging. Indeed, a successful vaccine requires, in addition to a high titer, establishment of long-term memory, production of neutralizing antibodies, lack of a cross-reaction, and needs to fulfill practical requirements for storage and handling (Lussow et al. 1990; Powell 1996). In addition, we found that whereas some amorphous aggregates are highly immunogenic, others are not (Kibria et al. 2021), strongly suggesting the need for further characterizing and classifying amorphous aggregates in classes according to biophysical properties other than size, as proposed by Narhi (2012). Nevertheless, we believe that SCP tags may be a useful tool to support the development of vaccines that use small globular proteins as immunogens without the need for additives to increase their immunogenicity (adjuvant-free).
Conclusion
Until recently, amorphous aggregation and low solubility were merely considered technical issues that must be solved to produce or use proteins. However, it is becoming increasingly clear that amorphous protein aggregation, including LLPS, may affect some physiological functions. These aggregates are thus receiving increased attention from a biophysical viewpoint, and it appears that amorphous aggregates can possess specific biophysical characteristics beside their sizes. Further investigations are obviously needed to link the biophysical attributes of aggregates and their physiological effects, especially the immunogenic responses they provoke. Finally, in this review, we showed that it is possible to control the formation of amorphous aggregates (oligomers) using SEP and SCP tags, and that they can be used to enhance the immunogenicity of recombinant proteins by controlling their aggregation. We hope that SEP and SCP tags will contribute to the development of protein subunit vaccines that are adjuvant-free (immunostimulant-free).
References
Arakawa T (2018) Protein–solvent interaction. Biophys Rev 10(2):203–208
Arakawa T, Gagnon P (2018) Excluded cosolvent in chromatography. J Pharm Sci 107(9):2297–2305
Baldwin RL, Rose GD (2016) How the hydrophobic factor drives protein folding. Proc Natl Acad Sci U S A 113(44):12462–12466
Boatz JC, Whitley MJ, Li M, Gronenborn AM, van der Wel PCA (2017) Cataract-associated P23T γD-crystallin retains a native-like fold in amorphous-looking aggregates formed at physiological pH. Nat Commun 5(8):15137
Brindha S, Kibria MG, Saotome T, Unzai S, Kuroda Y (2021) EGFR extracellular domain III expressed in Escherichia coli with SEP tag shows improved biophysical and functional properties and generates anti-sera inhibiting cancer cell growth. Biochem Biophys Res Commun 28(555):121–127
Brindha S, Kuroda Y (2022) A multi-disulfide receptor-binding domain (RBD) of the SARS-CoV-2 spike protein expressed in E. coli using a SEP-tag produces antisera interacting with the mammalian cell expressed spike (S1) protein. Int J Mol Sci 23(3):1703
Crill WD, Roehrig J (2001) Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75(16):7769–7773
Food and Drug Administration (FDA) (2014) Guidance for industry immunogenicity assessment for therapeutic protein products. https://www.fda.gov/media/85017/download
Gamble CN (1966) The role of soluble aggregates in the primary immune response of mice to human gamma globulin. Int Arch Allergy Appl Immunol 30(5):446–455
Hirota N, Edskes H, Hall D (2019) Unified theoretical description of the kinetics of protein aggregation. Biophys Rev 11(2):191–208
Islam MM, Khan MA, Kuroda Y (2012) Analysis of amino acid contributions to protein solubility using short peptide tags fused to a simplified BPTI variant. Biochim Biophys Acta 1824(10):1144–1150
Islam MM, Miura S et al (2020) Anti-dengue ED3 long-term immune response with T-cell memory generated using solubility controlling peptide tags. Front Immunol 11:333
Iwura T, Fukuda J, Yamazaki K, Arisaka F (2014) Conformational stability, reversibility and heat-induced aggregation of alpha-1-acid glycoprotein. J Biochem 156(6):345–352
Kabir GM, Islam MM, Kuroda Y (2018) Reversible association of proteins into sub-visible amorphous aggregates using short solubility controlling peptide tags. Biochim Biophys Acta 1866(2):366–372
Kato K, Maki T et al (2007) Mutational analysis of protein solubility enhancement using short peptide tags. Biopolymers 85:12–18
Khan MA, Islam MM, Kuroda Y (2013) Analysis of protein aggregation kinetics using short amino acid peptide tags. Biochim Biophys Acta 1834(10):2107–2115
Kibria MG, Akazawa-Ogawa Y, Rahman N, Hagihara Y, Kuroda Y (2020) The immunogenicity of an anti-EGFR single domain antibody (VHH) is enhanced by misfolded amorphous aggregation but not by heat-induced aggregation. Eur J Pharm Biopharm 152:164–174
Kibria MG, Fukutani A, Akazawa-Ogawa Y, Hagihara Y, Kuroda Y (2021) Anti-EGFR VHH antibody under thermal stress is better solubilized with a lysine than with an arginine SEP tag. Biomolecules 11(6):810
Kuroda Y (2018) Biophysical studies of protein solubility and aggregation by systematic mutational analysis and a helical polymerization model. Biophysical Review 10(2):473–480
Kuroda Y, Kim PS (2000) Folding of bovine pancreatic trypsin inhibitor (BPTI) variants in which almost half the residues are alanine. J Mol Biol 298(3):493–501
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157(1):105–132
Lussow AR, Aguado MT, Del Giudice G, Lambert PH (1990) Towards vaccine optimisation. Immunol Lett 25(1–3):255–263
Marszal E, Fowler E (2012) Workshop on predictive science of the immunogenicity aspects of particles in biopharmaceutical products. J Pharm Sci 101(10):3555–3559
Mitrea DM, Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14:1
Nakamura S, Kibria MG et al (2012) Reversible oligomerization and reverse hydrophobic effect induced by isoleucine tags attached at the C-terminus of a simplified BPTI variant. Biochemistry 59:3660–3668
Narhi LO, Schmit J, J. et al (2012) Classification of protein aggregates. J Pharm Sci 101(2):493–498
Nautiyal K, Kuroda Y (2018) A SEP tag enhances the expression, solubility and yield of recombinant TEV protease without altering its activity. N Biotechnol 25(42):77–84. https://doi.org/10.1016/j.nbt.2018.02.006
Nozaki Y, Tanford C (1971) The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem 246(7):2211–7
Onchaiya S, Saotome T, Mizutani K, Martinez JC, Tame JRH, Kidokoro SI, Kuroda Y (2022) Reverse engineering analysis of the high-temperature reversible oligomerization and amyloidogenicity of PSD95-PDZ3. Molecules 27(9):2813
Powell MF (1996) Drug delivery issues in vaccine development. Pharm Res 13(12):1777–1785
Rahman N, Islam MM et al (2020a) Nanometer-size aggregates generated using short solubility controlling peptide tags do increase the in vivo immunogenicity of a non-immunogenic protein. Mol Pharm 17(5):1629–1637
Rahman N, Islam MM et al (2020b) A systematic mutational analysis identifies a 5-residue proline tag that enhances the in vivo immunogenicity of a non-immunogenic model protein. FEBS Open 10(10):1947–1956
Rahman N, Miura S, Okawa M, Kibria MG, Islam MM, Kuroda Y (2021) Solubility controlling peptide tags of opposite charges generate a bivalent immune response against dengue ED3 serotypes 3 and 4. Front Immunol 11(12):671590
Ratanji KD, Derrick JP et al (2014) Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol 11(2):99–109
Ratanji KD, Dearman RJ, Kimber I, Thorpe R, Wadhwa M, Derrick JP (2016) Editor’s highlight: subvisible aggregates of immunogenic proteins promote a Th1-type response. Toxicol Sci 153(2):258–270
Rathnayaka T, Tawa M, Nakamura T, Sohya S, Kuwajima K, Yohda M, Kuroda Y (2011) Solubilization and folding of a fully active recombinant Gaussia luciferase with native disulfide bonds by using a SEP-Tag. Biochim Biophys Acta
Ripple DC, Dimitrova MN (2012) Protein particles: what we know and what we do not know. J Pharm Sci 101(10):3568–3579
Roberts CJ (2014) Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol 32(7):372–380
Saotome T, Nakamura S, Islam MM, Nakazawa A, Dellarole M, Arisaka F, Kidokoro S, Kuroda Y (2016) Unusual reversible oligomerization of unfolded dengue envelope protein domain 3 at high temperatures and its abolition by a point mutation. Biochemistry 55(32):4469–4475
Saotome T, Yamazaki T, Kuroda Y (2019) Misfolding of a single disulfide bonded globular protein into a low-solubility species conformationally and biophysically distinct from the native one. Biomolecules 9(6):250
Saotome T, Mezaki T, Brindha S, Unzai S, Martinez JC, Kidokoro SI, Kuroda Y (2020) Thermodynamic analysis of point mutations inhibiting high-temperature reversible oligomerization of PDZ3. Biophys J 119(7):1391–1401
Tanford C (1997) How protein chemists learned about the hydrophobic factor. Protein Sci 6(6):1358–1366
Timasheff SN (2002) Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry 41(46):13473–13482
Trevino SR, Scholtz JM, Pace CN (2007) Amino acid contribution to protein solubility: Asp, Glu, and Ser contribute more favorably than the other hydrophilic amino acids in RNase Sa. J Mol Biol 366(2):449–460
Uchiyama S, Noda M, Krayukhina E (2018) Sedimentation velocity analytical ultracentrifugation for characterization of therapeutic antibodies. Biophys Rev 10(2):259–269
Acknowledgements
This article is dedicated to Professor Emeritus Haruki Nakamura of Osaka University on the occasion of his 70th anniversary.
Funding
This work was supported by a JSPS grant-in-aid for scientific research (KAKENHI-15H04359 and 18H02385).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuroda, Y. Biophysical studies of amorphous protein aggregation and in vivo immunogenicity. Biophys Rev 14, 1495–1501 (2022). https://doi.org/10.1007/s12551-022-01011-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-022-01011-y