Abstract
Tropomodulins (Tmods) are proteins that cap the slow-growing (pointed) ends of actin filaments (F-actin). The basis for our current understanding of Tmod function comes from studies in cells with relatively stable and highly organized F-actin networks, leading to the view that Tmod capping functions principally to preserve F-actin stability. However, not only is Tmod capping dynamic, but it also can play major roles in regulating diverse cellular processes involving F-actin remodeling. Here, we highlight the multifunctional roles of Tmod with a focus on Tmod3. Like other Tmods, Tmod3 binds tropomyosin (Tpm) and actin, capping pure F-actin at submicromolar and Tpm-coated F-actin at nanomolar concentrations. Unlike other Tmods, Tmod3 can also bind actin monomers and its ability to bind actin is inhibited by phosphorylation of Tmod3 by Akt2. Tmod3 is ubiquitously expressed and is present in a diverse array of cytoskeletal structures, including contractile structures such as sarcomere-like units of actomyosin stress fibers and in the F-actin network encompassing adherens junctions. Tmod3 participates in F-actin network remodeling in lamellipodia during cell migration and in the assembly of specialized F-actin networks during exocytosis. Furthermore, Tmod3 is required for development, regulating F-actin mesh formation during meiosis I of mouse oocytes, erythroblast enucleation in definitive erythropoiesis, and megakaryocyte morphogenesis in the mouse fetal liver. Thus, Tmod3 plays vital roles in dynamic and stable F-actin networks in cell physiology and development, with further research required to delineate the mechanistic details of Tmod3 regulation in the aforementioned processes, or in other yet to be discovered processes.


Similar content being viewed by others
References
Abercrombie M, Heaysman JE, Pegrum SM (1970) The locomotion of fibroblasts in culture. II. “RRuffling”. Exp Cell Res 60:437–444
Almonacid M, Terret ME, Verlhac MH (2014) Actin-based spindle positioning: new insights from female gametes. J Cell Sci 127:477–483. https://doi.org/10.1242/jcs.142711
Almonacid M, Terret ME, Verlhac MH (2018) Control of nucleus positioning in mouse oocytes. Semin Cell Dev Biol 82:34–40
Barua B, Nagy A, Sellers JR, Hitchcock-DeGregori SE (2014) Regulation of nonmuscle myosin II by tropomyosin. Biochemistry 53:4015–4024. https://doi.org/10.1021/bi500162z
Bennett V, Healy J (2009) Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol 1:a003012. https://doi.org/10.1101/cshperspect.a003012
Blanchoin L, Pollard TD, Hitchcock-DeGregori SE (2001) Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin. Curr Biol 11:1300–1304
Brayford S, Bryce NS, Schevzov G, Haynes EM, Bear JE, Hardeman EC, Gunning PW (2016) Tropomyosin promotes lamellipodial persistence by collaborating with Arp2/3 at the leading edge. Curr Biol 26:1312–1318. https://doi.org/10.1016/j.cub.2016.03.028
Caldwell BJ et al (2014) Tropomyosin isoforms support actomyosin biogenesis to generate contractile tension at the epithelial zonula adherens. Cytoskeleton (Hoboken) 71:663–676. https://doi.org/10.1002/cm.21202
Carlier MF et al (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136:1307–1322
Carlier MF, Shekhar S (2017) Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat Rev Mol Cell Biol 18:389–401. https://doi.org/10.1038/nrm.2016.172
Charras G, Yap AS (2018) Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol 28:R445–R457. https://doi.org/10.1016/j.cub.2018.02.003
Chiu TT, Patel N, Shaw AE, Bamburg JR, Klip A (2010) Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol Biol Cell 21:3529–3539. https://doi.org/10.1091/mbc.E10-04-0316
Chung le TK et al (2010) Myosin IIA participates in docking of Glut4 storage vesicles with the plasma membrane in 3T3-L1 adipocytes. Biochem Biophys Res Commun 391:995–999. https://doi.org/10.1016/j.bbrc.2009.12.004
Clayton JE, Pollard LW, Murray GG, Lord M (2015) Myosin motor isoforms direct specification of actomyosin function by tropomyosins. Cytoskeleton (Hoboken) 72:131–145. https://doi.org/10.1002/cm.21213
Colpan M, Moroz NA, Gray KT, Cooper DA, Diaz CA, Kostyukova AS (2016) Tropomyosin-binding properties modulate competition between tropomodulin isoforms. Arch Biochem Biophys 600:23–32. https://doi.org/10.1016/j.abb.2016.04.006
Colpan M, Moroz NA, Kostyukova AS (2013) Tropomodulins and tropomyosins: working as a team. J Muscle Res Cell Motil 34:247–260. https://doi.org/10.1007/s10974-013-9349-6
Cox-Paulson E et al (2014) The minus-end actin capping protein, UNC-94/tropomodulin, regulates development of the Caenorhabditis elegans intestine. Dev Dyn 243:753–764. https://doi.org/10.1002/dvdy.24118
Cox-Paulson EA et al (2012) Tropomodulin protects alpha-catenin-dependent junctional-actin networks under stress during epithelial morphogenesis. Curr Biol 22:1500–1505. https://doi.org/10.1016/j.cub.2012.06.025
Cox PR, Zoghbi HY (2000) Sequencing, expression analysis, and mapping of three unique human tropomodulin genes and their mouse orthologs. Genomics 63:97–107. https://doi.org/10.1006/geno.1999.6061
DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE (2002) Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J Cell Sci 115:4649–4660
Ebrahim S et al (2013) NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol 23:731–736. https://doi.org/10.1016/j.cub.2013.03.039
Fischer RS, Fowler VM (2003) Tropomodulins: life at the slow end. Trends Cell Biol 13:593–601
Fischer RS, Fritz-Six KL, Fowler VM (2003) Pointed-end capping by tropomodulin3 negatively regulates endothelial cell motility. J Cell Biol 161:371–380. https://doi.org/10.1083/jcb.200209057
Fischer RS, Yarmola EG, Weber KL, Speicher KD, Speicher DW, Bubb MR, Fowler VM (2006) Tropomodulin 3 binds to actin monomers. J Biol Chem 281:36454–36465. https://doi.org/10.1074/jbc.M606315200
Fowler VM (1987) Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. J Biol Chem 262:12792–12800
Fowler VM (1996) Regulation of actin filament length in erythrocytes and striated muscle. Curr Opin Cell Biol 8:86–96
Fowler VM, Dominguez R (2017) Tropomodulins and leiomodins: actin pointed end caps and Nucleators in muscles. Biophys J 112:1742–1760. https://doi.org/10.1016/j.bpj.2017.03.034
Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP (1993) Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J Cell Biol 120:411–420
Gajbhiye R et al (2017) Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod Sci 24:413–420. https://doi.org/10.1177/1933719116657190
Gajbhiye R et al (2012) Identification and validation of novel serum markers for early diagnosis of endometriosis. Hum Reprod 27:408–417. https://doi.org/10.1093/humrep/der410
Gateva G et al (2017) Tropomyosin isoforms specify functionally distinct actin filament populations in vitro. Curr Biol 27:705–713. https://doi.org/10.1016/j.cub.2017.01.018
Gokhin DS, Fowler VM (2011a) Cytoplasmic gamma-actin and tropomodulin isoforms link to the sarcoplasmic reticulum in skeletal muscle fibers. J Cell Biol 194:105–120. https://doi.org/10.1083/jcb.201011128
Gokhin DS, Fowler VM (2011b) The sarcoplasmic reticulum: actin and tropomodulin hit the links. Bioarchitecture 1:175–179. https://doi.org/10.4161/bioa.1.4.17533
Gokhin DS, Fowler VM (2011c) Tropomodulin capping of actin filaments in striated muscle development and physiology. J Biomed Biotechnol 2011:103069. https://doi.org/10.1155/2011/103069
Gokhin DS, Fowler VM (2013) A two-segment model for thin filament architecture in skeletal muscle. Nat Rev Mol Cell Biol 14:113–119. https://doi.org/10.1038/nrm3510
Gokhin DS, Fowler VM (2016) Feisty filaments: actin dynamics in the red blood cell membrane skeleton. Curr Opin Hematol 23:206–214. https://doi.org/10.1097/MOH.0000000000000227
Gokhin DS et al (2010) Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J Cell Biol 189:95–109. https://doi.org/10.1083/jcb.201001125
Gonzalez E, McGraw TE (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8:2502–2508. https://doi.org/10.4161/cc.8.16.9335
Gray KT, Kostyukova AS, Fath T (2017) Actin regulation by tropomodulin and tropomyosin in neuronal morphogenesis and function. Mol Cell Neurosci 84:48–57. https://doi.org/10.1016/j.mcn.2017.04.002
Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM (1995) Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature 377:83–86. https://doi.org/10.1038/377083a0
Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R (2014) E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal 7:rs7. https://doi.org/10.1126/scisignal.2005473
Gupton SL et al (2005) Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol 168:619–631. https://doi.org/10.1083/jcb.200406063
Hertzog M et al (2004) The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell 117:611–623
Hillberg L, Zhao Rathje LS, Nyakern-Meazza M, Helfand B, Goldman RD, Schutt CE, Lindberg U (2006) Tropomyosins are present in lamellipodia of motile cells. Eur J Cell Biol 85:399–409. https://doi.org/10.1016/j.ejcb.2005.12.005
Hu S et al (2017) Erratum: long-range self-organization of cytoskeletal myosin II filament stacks. Nat Cell Biol 19:258. https://doi.org/10.1038/ncb3479
Ji P, Murata-Hori M, Lodish HF (2011) Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol 21:409–415. https://doi.org/10.1016/j.tcb.2011.04.003
Jo YJ, Jang WI, Kim NH, Namgoong S (2016) Tropomodulin-3 is essential in asymmetric division during mouse oocyte maturation. Sci Rep 6:29204. https://doi.org/10.1038/srep29204
Kee AJ et al (2015) An actin filament population defined by the tropomyosin Tpm3.1 regulates glucose uptake. Traffic 16:691–711. https://doi.org/10.1111/tra.12282
Lecuit T, Yap AS (2015) E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol 17:533–539. https://doi.org/10.1038/ncb3136
Lee CW, Vitriol EA, Shim S, Wise AL, Velayutham RP, Zheng JQ (2013) Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr Biol 23:1046–1056. https://doi.org/10.1016/j.cub.2013.04.057
Lewis RA, Yamashiro S, Gokhin DS, Fowler VM (2014) Functional effects of mutations in the tropomyosin-binding sites of tropomodulin1 and tropomodulin3. Cytoskeleton (Hoboken) 71:395–411. https://doi.org/10.1002/cm.21179
Li R (2013) The art of choreographing asymmetric cell division. Dev Cell 25:439–450. https://doi.org/10.1016/j.devcel.2013.05.003
Lim CY, Bi X, Wu D, Kim JB, Gunning PW, Hong W, Han W (2015) Tropomodulin3 is a novel Akt2 effector regulating insulin-stimulated GLUT4 exocytosis through cortical actin remodeling. Nat Commun 6:5951. https://doi.org/10.1038/ncomms6951
Littlefield R, Almenar-Queralt A, Fowler VM (2001) Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol 3:544–551. https://doi.org/10.1038/35078517
Lopez-Ubeda R, Garcia-Vazquez FA, Romar R, Gadea J, Munoz M, Hunter RH, Coy P (2015) Oviductal transcriptome is modified after insemination during spontaneous ovulation in the sow. PLoS One 10:e0130128. https://doi.org/10.1371/journal.pone.0130128
Lopez JA et al (2009) Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell 20:3918–3929. https://doi.org/10.1091/mbc.E09-03-0187
Lu Y, Ye Y, Bao W, Yang Q, Wang J, Liu Z, Shi S (2017) Genome-wide identification of genes essential for podocyte cytoskeletons based on single-cell RNA sequencing. Kidney Int 92:1119–1129. https://doi.org/10.1016/j.kint.2017.04.022
McKeown CR, Nowak RB, Gokhin DS, Fowler VM (2014) Tropomyosin is required for cardiac morphogenesis, myofibril assembly, and formation of adherens junctions in the developing mouse embryo. Dev Dyn 243:800–817. https://doi.org/10.1002/dvdy.24115
Mege RM, Ishiyama N (2017) Integration of cadherin adhesion and cytoskeleton at Adherens junctions Cold Spring Harb Perspect Biol 9 https://doi.org/10.1101/cshperspect.a028738
Miklavc P, Ehinger K, Sultan A, Felder T, Paul P, Gottschalk KE, Frick M (2015) Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J Cell Sci 128:1193–1203. https://doi.org/10.1242/jcs.165571
Miklavc P, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C, Frick M (2012) Actin coating and compression of fused secretory vesicles are essential for surfactant secretion--a role for Rho, formins and myosin II. J Cell Sci 125:2765–2774. https://doi.org/10.1242/jcs.105262
Moyer JD et al (2010) Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton. Blood 116:2590–2599. https://doi.org/10.1182/blood-2010-02-268458
Mullins RD, Heuser JA, Pollard TD (1998) The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A 95:6181–6186
Namgoong S, Kim NH (2016) Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle 15:1830–1843. https://doi.org/10.1080/15384101.2016.1181239
Naumanen P, Lappalainen P, Hotulainen P (2008) Mechanisms of actin stress fibre assembly. J Microsc 231:446–454. https://doi.org/10.1111/j.1365-2818.2008.02057.x
Nowak RB et al (2017) Tropomodulin 1 controls erythroblast enucleation via regulation of F-actin in the enucleosome. Blood 130:1144–1155. https://doi.org/10.1182/blood-2017-05-787051
Ochala J, Gokhin DS, Iwamoto H, Fowler VM (2014) Pointed-end capping by tropomodulin modulates actomyosin crossbridge formation in skeletal muscle fibers. FASEB J 28:408–415. https://doi.org/10.1096/fj.13-239640
Ojala PJ, Paavilainen VO, Vartiainen MK, Tuma R, Weeds AG, Lappalainen P (2002) The two ADF-H domains of twinfilin play functionally distinct roles in interactions with actin monomers. Mol Biol Cell 13:3811–3821. https://doi.org/10.1091/mbc.e02-03-0157
Ono S (2010) Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton (Hoboken) 67:677–692. https://doi.org/10.1002/cm.20476
Paez AV et al (2016) Heme oxygenase-1 in the forefront of a multi-molecular network that governs cell-cell contacts and filopodia-induced zippering in prostate cancer. Cell Death Dis 7:e2570. https://doi.org/10.1038/cddis.2016.420
Patel-Hett S et al (2011) The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood 118:1641–1652. https://doi.org/10.1182/blood-2011-01-330688
Pathan-Chhatbar S, Taft MH, Reindl T, Hundt N, Latham SL, Manstein DJ (2018) Three mammalian tropomyosin isoforms have different regulatory effects on nonmuscle myosin-2B and filamentous beta-actin in vitro. J Biol Chem 293:863–875. https://doi.org/10.1074/jbc.M117.806521
Pellegrin S, Mellor H (2007) Actin stress fibres. J Cell Sci 120:3491–3499. https://doi.org/10.1242/jcs.018473
Pilot F, Lecuit T (2005) Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn 232:685–694. https://doi.org/10.1002/dvdy.20334
Pollard TD, Blanchoin L, Mullins RD (2000) Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct 29:545–576. https://doi.org/10.1146/annurev.biophys.29.1.545
Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112:453–465
Pollard TD, Cooper JA (2009) Actin, a central player in cell shape and movement. Science 326:1208–1212. https://doi.org/10.1126/science.1175862
Qiu J et al (2010) Gene expression profiles of adipose tissue of high-fat diet-induced obese rats by cDNA microarrays. Mol Biol Rep 37:3691–3695. https://doi.org/10.1007/s11033-010-0021-6
Rao JN, Madasu Y, Dominguez R (2014) Mechanism of actin filament pointed-end capping by tropomodulin. Science 345:463–467. https://doi.org/10.1126/science.1256159
Ridley AJ (2011) Life at the leading edge. Cell 145:1012–1022. https://doi.org/10.1016/j.cell.2011.06.010
Stevenson TO, Mercer KB, Cox EA, Szewczyk NJ, Conley CA, Hardin JD, Benian GM (2007) unc-94 encodes a tropomodulin in Caenorhabditis elegans. J Mol Biol 374:936–950. https://doi.org/10.1016/j.jmb.2007.10.005
Sui Z et al (2014) Tropomodulin3-null mice are embryonic lethal with anemia due to impaired erythroid terminal differentiation in the fetal liver. Blood 123:758–767. https://doi.org/10.1182/blood-2013-03-492710
Sui Z, Nowak RB, Sanada C, Halene S, Krause DS, Fowler VM (2015) Regulation of actin polymerization by tropomodulin-3 controls megakaryocyte actin organization and platelet biogenesis. Blood 126:520–530. https://doi.org/10.1182/blood-2014-09-601484
Tran DT, Masedunskas A, Weigert R, Ten Hagen KG (2015) Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat Commun 6:10098. https://doi.org/10.1038/ncomms10098
Uversky VN, Shah SP, Gritsyna Y, Hitchcock-DeGregori SE, Kostyukova AS (2011) Systematic analysis of tropomodulin/tropomyosin interactions uncovers fine-tuned binding specificity of intrinsically disordered proteins. J Mol Recognit 24:647–655. https://doi.org/10.1002/jmr.1093
Vitriol EA, McMillen LM, Kapustina M, Gomez SM, Vavylonis D, Zheng JQ (2015) Two functionally distinct sources of actin monomers supply the leading edge of lamellipodia. Cell Rep 11:433–445. https://doi.org/10.1016/j.celrep.2015.03.033
Vuong-Brender TTK, Boutillon A, Rodriguez D, Lavilley V, Labouesse M (2018) HMP-1/alpha-catenin promotes junctional mechanical integrity during morphogenesis. PLoS One 13:e0193279. https://doi.org/10.1371/journal.pone.0193279
Weber A, Pennise CR, Babcock GG, Fowler VM (1994) Tropomodulin caps the pointed ends of actin filaments. J Cell Biol 127:1627–1635
Weber KL, Fischer RS, Fowler VM (2007) Tmod3 regulates polarized epithelial cell morphology. J Cell Sci 120:3625–3632. https://doi.org/10.1242/jcs.011445
Yamashiro S, Gokhin DS, Kimura S, Nowak RB, Fowler VM (2012) Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton (Hoboken) 69:337–370. https://doi.org/10.1002/cm.21031
Yamashiro S, Gokhin DS, Sui Z, Bergeron SE, Rubenstein PA, Fowler VM (2014) Differential actin-regulatory activities of Tropomodulin1 and Tropomodulin3 with diverse tropomyosin and actin isoforms. J Biol Chem 289:11616–11629. https://doi.org/10.1074/jbc.M114.555128
Yamashiro S, Speicher KD, Speicher DW, Fowler VM (2010) Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities. J Biol Chem 285:33265–33280. https://doi.org/10.1074/jbc.M110.144873
Yang W et al (2014) Arp2/3 complex regulates adipogenesis by controlling cortical actin remodelling. Biochem J 464:179–192. https://doi.org/10.1042/BJ20140805
Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R (2011) Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 13:1252–1258. https://doi.org/10.1038/ncb2320
Yu FX, Lin SC, Morrison-Bogorad M, Atkinson MA, Yin HL (1993) Thymosin beta 10 and thymosin beta 4 are both actin monomer sequestering proteins. J Biol Chem 268:502–509
Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM (2005) Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci 118:5549–5562. https://doi.org/10.1242/jcs.02639
Acknowledgements
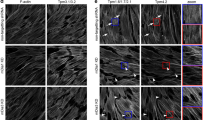
We are grateful to Roberta B. Nowak for the preparation of Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding information
This work was funded by the National Institutes of Health (NIH) Grants R01 HL083464 and R01 EY017724 to V.M.F. J.P. was supported by a fellowship from the Natural Science and Engineering Research Council of Canada.
Conflict of interest
Justin Parreno declares that he has no conflict of interest. Velia M. Fowler declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Parreno, J., Fowler, V.M. Multifunctional roles of tropomodulin-3 in regulating actin dynamics. Biophys Rev 10, 1605–1615 (2018). https://doi.org/10.1007/s12551-018-0481-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-018-0481-9