Abstract
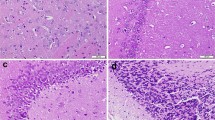
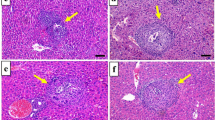
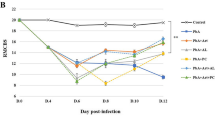
Populations in malaria endemic areas are frequently exposed to mycotoxin-contaminated diets. The possible toxicological outcome of co-exposure to dietary aflatoxin B1 (AFB1) and artemisinin-based combination therapy warrants investigation to ascertain amplification or attenuation of cellular injury. Here, we investigated the neurobehavioral and biochemical responses associated with co-exposure to anti-malarial drug coartem, an artemether-lumefantrine combination (5 mg/kg body weight, twice a day and 3 days per week) and AFB1 (35 and 70 µg/kg body weight) in rats. Motor deficits, locomotor incompetence, and anxiogenic-like behavior induced by low AFB1 dose were significantly (p < 0.05) assuaged by coartem but failed to rescue these behavioral abnormalities in high AFB1-dosed group. Coartem administration did not alter exploratory deficits typified by reduced track plot densities and greater heat map intensity in high AFB1-dosed animals. Furthermore, the reduction in cerebral and cerebellar acetylcholinesterase activity, anti-oxidant enzyme activities, and glutathione and thiol levels were markedly assuaged by coartem administration in low AFB1 group but not in high AFB1-dosed animals. The significant attenuation of cerebral and cerebellar oxidative stress indices namely reactive oxygen and nitrogen species, xanthine oxidase activity, and lipid peroxidation by coartem administration was evident in low AFB1 group but not high AFB1 dose. Although coartem administration abated nitric oxide level, activities of myeloperoxidase, caspase-9, and caspase-3 in animals exposed to both doses of AFB1, these indices were significantly higher than the control. Coartem administration ameliorated histopathological and mophometrical changes due to low AFB1 exposure but not in high AFB1 exposure. In conclusion, contrary to AFB1 alone, behavioral and biochemical responses were not altered in animals singly exposed to coartem. Co-exposure to coartem and AFB1 elicited no additional risk but partially lessened neurotoxicity associated with AFB1 exposure.










Similar content being viewed by others
Data availability
The original data and materials of the current study are available with the corresponding author and would be made available on justifiable request.
References
Adedara IA, Abolaji AO, Awogbindin IO, Farombi EO (2017a) Suppression of the brain-pituitary-testicular axis function following acute arsenic and manganese co-exposure and withdrawal in rats. J Trace Elem Med Biol 39:21–29
Adedara IA, Abolaji AO, Idris UF, Olabiyi BF, Onibiyo EM, Ojuade TD, Farombi EO (2017b) Neuroprotective influence of taurine on fluoride-induced biochemical and behavioral deficits in rats. Chem Biol Interact 261:1–10
Adedara IA, Abolaji AO, Rocha JB, Farombi EO (2016) Diphenyl diselenide protects against mortality, locomotor deficits and oxidative stress in Drosophila melanogaster model of manganese-induced neurotoxicity. Neurochem Res 41:1430–1438
Adedara IA, Fasina OB, Ayeni MF, Ajayi OM, Farombi EO (2019) Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem Toxicol 125:170–181
Adedara IA, Nanjappa MK, Farombi EO, Akingbemi BT (2014) Aflatoxin B1 disrupts the androgen biosynthetic pathway in rat Leydig cells. Food Chem Toxicol 65:252–259
Adedara IA, Owumi SE, Oyelere AK, Farombi EO (2021) Neuroprotective role of gallic acid in aflatoxin B1-induced behavioral abnormalities in rats. J Biochem Mol Toxicol 35:e22684. https://doi.org/10.1002/jbt.22684
Allen SJ, Wild CP, Wheeler JG, Riley EM, Montesano R, Bennett S, Whittle HC, Hall AJ, Greenwood BM (1992) Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children. Trans R Soc Trop Med Hyg 86:426–430
Aytekin Sahin G, Karabulut D, Unal G, Sayan M, Sahin H (2022) Effects of probiotic supplementation on very low dose AFB1-induced neurotoxicity in adult male rats. Life Sci 306:120798. https://doi.org/10.1016/j.lfs.2022.120798
Bahey NG, Abd Elaziz HO, Gadalla KK (2015) Toxic effect of aflatoxin B1 and the role of recovery on the rat cerebral cortex and hippocampus. Tissue Cell 47:559–566
Baldissera MD, Souza CF, Zeppenfeld CC, Descovi SN, Moreira KLS, da Rocha MIUM, da Veiga ML, da Silva AS, Baldisserotto B (2018) Aflatoxin B1-contaminated diet disrupts the blood-brain barrier and affects fish behavior: involvement of neurotransmitters in brain synaptosomes. Environ Toxicol Pharmacol 60:45–51
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana SI, Yamauchi M, Opio W, Emoto S, Anywar DA, Kimura E, Palacpac NMQ, Odongo-Aginya EI, Ogwang M, Horii T, Mita T (2021) Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385(13):1163–1171
Bancroft JD, Gamble M (2008) Theory and practice of histology techniques, 6th ed. Churchill Livingstone Elsevier, pp. 83–134
Benkerroum N (2020) Aflatoxins: producing-molds, structure, health issues and incidence in Southeast Asian and Sub-Saharan African countries. Int J Environ Res Public Health 17:1215
Bingman VP, Sharp PE (2006) Neuronal implementation of hippocampal-mediated spatial behavior: a comparative evolutionary perspective. Behav Cogn Neurosci Rev 5:80–91
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cenini G, Lloret A, Cascella R (2019) Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid Med Cell Longev 2019:2105607. https://doi.org/10.1155/2019/2105607
Claiborne A (1995) Catalase activity. In: Greewald AR (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 237–242
Classen W, Altmann B, Gretener P, Souppart C, Skelton-Stroud P, Krinke G (1999) Differential effects of orally versus parenterally administered qinghaosu derivatives artemether in dogs. Exp Toxicol Pathol 51:507–516
El-Dakdoky MH (2009) Evaluation of the developmental toxicity of artemether during different phases of rat pregnancy. Food Chem Toxicol 47:1437–1441
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ferrari L, Fumagalli F, Rizzi N, Grandi E, Vailati S, Manoni M, Ottoboni M, Cheli F, Pinotti L (2022) An eight-year survey on aflatoxin B1 indicates high feed safety in animal feed and forages in Northern Italy. Toxins (basel) 14(11):763. https://doi.org/10.3390/toxins14110763
Folarin O, Olopade F, Onwuka S, Olopade J (2016) Memory deficit recovery after chronic vanadium exposure in mice. Oxid Med Cell Longev 2016:4860582. https://doi.org/10.1155/2016/4860582
Gautam A, Ahmed T, Batra V, Paliwal J (2009) Pharmacokinetics and pharmacodynamics of endoperoxide antimalarials. Curr Drug Metab 10:289–306
Gorny JH, Gorny B, Wallace DG, Whishaw IQ (2002) Fimbria-fornix lesions disrupt the dead reckoning (homing) component of exploratory behavior in mice. Learn Mem 9:387–394
Granell S, Gironella M, Bulbena O, Panés J, Mauri M, Sabater L, Aparisi L, Gelpí E, Closa D (2003) Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med 31:525–530
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hernandez Maldonado J, Grundmann O (2022) Drug-drug interactions of artemisinin-based combination therapies in malaria treatment: a narrative review of the literature. J Clin Pharmacol 62:1197–1205
Ho WE, Peh HY, Chan TK, Wong WS (2014) Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 142:126–139
International Agency for Research on Cancer (2000) Monographs on aflatoxins, vol 82. IARC Press, Lyon, pp 171–300
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kamchonwongpaisan S, McKeever P, Hossler P, Ziffer H, Meshnick SR (1997) Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg 56:7–12
Kapil V, Khambata RS, Jones DA, Rathod K, Primus C, Massimo G, Fukuto JM, Ahluwalia A (2020) The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev 72:692–766
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48:203–213
Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brümmer J, Rudolph V, Münzel T, Heitzer T, Meinertz T, Baldus S (2005) Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA 102:431–436
Le Ray D, Bertrand SS, Dubuc R (2022) Cholinergic modulation of locomotor circuits in vertebrates. Int J Mol Sci 23:10738. https://doi.org/10.3390/ijms231810738
Li Q, Hickman M (2011) Toxicokinetic and toxicodynamic (TK/TD) evaluation to determine and predict the neurotoxicity of artemisinins. Toxicology 279(1–3):1–9
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237
Meng Y, Ma N, Lyu H, Wong YK, Zhang X, Zhu Y, Gao P, Sun P, Song Y, Lin L, Wang J (2021) Recent pharmacological advances in the repurposing of artemisinin drugs. Med Res Rev 41:3156–3181
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Motz BA, Alberts JR (2005) The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol 27:529–533
Nuwagira C, Peter EL, Ajayi CO, Adriko J, Kagoro GR, Olet EA, Ogwang PE, Tolo CU (2021) Developmental stages influence in vivo antimalarial activity of aerial part extracts of Schkuhria pinnata. J Ethnopharmacol 279:114341. https://doi.org/10.1016/j.jep.2021.114341
Owoeye O, Adedara IA, Farombi EO (2018) Pretreatment with taurine prevented brain injury and exploratory behaviour associated with administration of anticancer drug cisplatin in rats. Biomed Pharmacother 102:375–384
Owumi SE, Adedara IA, Oyelere AK (2022) Indole-3-propionic acid mitigates chlorpyrifos-mediated neurotoxicity by modulating cholinergic and redox-regulatory systems, inflammatory stress, apoptotic responses and DNA damage in rats, Environ. Toxicol Pharmacol 89:103786. https://doi.org/10.1016/j.etap.2021.103786
Owumi SE, Gbadegesin MA, Odunola OA, Adegoke AM, Uwaifo AO (2015) Toxicity associated with repeated administration of artemether-lumefantrine in rats. Environ Toxicol 30:301–307
Pernaute-Lau L, Camara M, Nóbrega de Sousa T, Morris U, Ferreira MU, Gil JP (2022) An update on pharmacogenetic factors influencing the metabolism and toxicity of artemisinin-based combination therapy in the treatment of malaria. Expert Opin Drug Metab Toxicol 18:39–59
Petras JM, Young GD, Bauman RA, Kyle DE, Gettayacamin M, Webster HK, Corcoran KD, Peggins JO, Vane MA, Brewer TG (2000) Brewer, Arteether-induced brain injury in Macaca mulatta I. The precerebellar nuclei: the lateral reticular nuclei, paramedian reticular nuclei, and perihypoglossal nuclei. Anat Embryol (berl) 201:383–397
Qureshi H, Hamid SS, Ali SS, Anwar J, Siddiqui AA, Khan NA (2015) Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood-brain barrier. Med Mycol 53:409–416
Ramos-Martín V, González-Martínez C, Mackenzie I, Schmutzhard J, Pace C, Lalloo DG, Terlouw DJ (2014) Neuroauditory toxicity of artemisinin combination therapies-have safety concerns been addressed? Am J Trop Med Hyg 91:62–73
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Rushing BR, Selim MI (2019) Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 124:81–100
Skelton-Stroud P, Mull R (1998) Positioning, labelling, and medical information control of co-artemether tablets (CPG 56697): a fixed novel combination of artemether and benflumetol. Novartis Co-Artemether International Development Team. Med Trop (mars) 58:77–81
Souto NS, Claudia Monteiro Braga A, Lutchemeyer de Freitas M, Rechia Fighera M, Royes LFF, Schneider Oliveira M, Furian AF (2018) Aflatoxin B1 reduces non-enzymatic antioxidant defenses and increases protein kinase C activation in the cerebral cortex of young rats. Nutr Neurosci 21:268–275
Sullivan AP (2022) Mycotoxin illness: recognition and management from functional medicine perspective. Phys Med Rehabil Clin N Am 33:647–663
Tanaka T, Mizukami S, Hasegawa-Baba Y, Onda N, Sugita-Konishi Y, Yoshida T, Shibutani M (2015) Developmental exposure of aflatoxin B1 reversibly affects hippocampal neurogenesis targeting late-stage neural progenitor cells through suppression of cholinergic signaling in rats. Toxicology 336:59–69
Unnisa A, Greig NH, Kamal MA (2022) Inhibition of caspase 3 and caspase 9 mediated apoptosis: a multimodal therapeutic target in traumatic brain injury. Curr Neuropharmacol 27. https://doi.org/10.2174/1570159X20666220327222921
van Koppen CJ, Kaiser B (2003) Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther 98:197–220
Wagacha JM, Muthomi JW (2008) Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol 124:1–12
World Health Organization (2020) World Malaria Report. www.who.int/publications/i/item/9789240015791
Wu TS, Cheng YC, Chen PJ, Huang YT, Yu FY, Liu BH (2019) Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere 217:905–913
Zhang T, Zhang X, Lin C, Wu S, Wang F, Wang H, Wang Y, Peng Y, Hutchinson MR, Li H, Wang X (2021) Artemisinin inhibits TLR4 signaling by targeting co-receptor MD2 in microglial BV-2 cells and prevents lipopolysaccharide-induced blood-brain barrier leakage in mice. J Neurochem 157:611–623
Zimcikova E, Simko J, Karesova I, Kremlacek J, Malakova J (2017) Behavioral effects of antiepileptic drugs in rats: are the effects on mood and behavior detectable in open-field test? Seizure 52:35–40
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adedara, I.A., Owumi, S.E. Neurobehavioral and biochemical responses to artemisinin-based drug and aflatoxin B1 co-exposure in rats. Mycotoxin Res 39, 67–80 (2023). https://doi.org/10.1007/s12550-023-00474-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-023-00474-6