Abstract
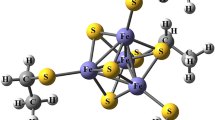
As a continuation of our previous work (de Courcy et al., 2008. J. Chem. Theo. Comput. 4 1659), lone pair-cation interactions were quantum-mechanically studied within the active site of the alcohol dehydrogenase Zn(II)-metalloenzyme by means of the topological analysis of the Electron Localization Function (ELF) and the Reduced Variational Space (RVS) energy decomposition analysis. Ligands lone pairs in direct interaction with the metal were shown to control the physical nature of the interaction as it appears to be dominated by polarization when the number of interacting lone pairs increases. Furthermore, we observed a peculiar behaviour of the cysteinate S− lone pairs which can redistribute and merge, thereby reducing their number to accommodate the zinc cation which also exhibits a consequent plasticity of its density outer shells which can delocalize towards ligands. Such observations should allow a deeper understanding of the usual softness/hardness concept of ions and ligands.
Similar content being viewed by others
References
Bader, R.F.W 1990. Atoms in Molecules-A Quantum Theory. Oxford University Press, Oxford.
Becke, A.D 1988. Correlation energy of an inhomogeneous electron gas: A coordinate-space model. J Chem Phys 88, 1053–1062.
Becke, A.D., Edgecombe, K.E 1990. A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92, 5397–5403.
Crow, K.E., Hardman, M.J 1989. Regulation of rates of ethanol metabolism. In: Crow, K.E., Batt, R.D. (eds) Human Metabolism of Alcohol, Vol. 2. CRC Press, Boca Raton, 3–16.
de Courcy, B., Piquemal, J.-P., Gresh, N. 2008. Energy analysis of Zn polycoordination in a metalloprotein environment and of the role of a neighboring aromatic residue. What is the impact of polarization? J Chem Theo Comput 4, 1659–1668.
Dudev, T., Lim, C. 2003. Principles governing Mg, Ca, and Zn binding and selectivities in proteins. Chem Rev 103, 773–788.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toy-ota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clirord, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A. 2007. Gaussian 03, Revision C.02. Gaussian Inc., Wallingford, CT.
Garmer, D.R., Gresh, N 1994. A comprehensive energy component analysis of the interaction of hard and soft dications with biological ligands. J Am Chem Soc 116, 3556–3567.
Gillespie, R.J., Robinson, E.A. 2005. Models of molecular geometry. Chem Soc Rev 34, 396–407.
Gourlaouen, C., Gérard, H., Piquemal J.-P., Parisel, O. 2008. Understanding lead chemistry from topological insights: the transition between holo- and hemidirected structures within the [Pb(CO)n]2+ model series. Chem Eur J 14, 2730–2743.
Gresh, N, Stevens, W.J., Krauss, M 1995. Mono- and polyligated complexes of Zn2+. An ab initio analysis of the metal-ligand interaction energy. J Comput Chem 16, 843–855.
Gresh, N 1995. Energetics of Zn2+ binding to a series of biologically-relevant ligands. A molecular mechanics investigation grounded on ab initio SCF supermolecular computations. J Comput Chem 16, 856–882.
Gresh, N., Cisneros, Darden, T.A., Piquemal, J.-P. 2007. Anisotropic, polarizable molecular mechanics studies of inter-, intra-molecular interactions, and ligand-macromolecule complexes. A bottom-up strategy. J Chem Theory Comput 3, 1960–1986.
Krishnan, R., Binkley, J.S., Seeger, R., Pople, J.A 1980. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72, 650–654.
Lee, C., Yang, W., Parr, R.G 1988. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37, 785–789.
Li, H., Hallows, W.H., Punzi, J.S., Pankiewicz, K.W., Watanabe, K.A., Goldstein, B.M 1994. Crystallographic Studies of Isosteric NAD Analogues Bound to Alcohol Dehydrogenase: Specificity and Substrate Binding in Two Ternary Complexes. Biochemistry 33, 11734–11744.
Maynard, A.T., Covell, D.G. 2001. Reactivity of Zinc Finger core: analysis of protein packing and electrostatic screening. J Am Chem Soc 123, 1047–1058.
McLean, A.D., Chandler, G.S 1980. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18. J Chem Phys 72, 5639–5648.
Noury, S., Krokidis, X., Fuster, F., Silvi, B 1999. Computational tools for the electron localization function topological analysis. Comput Chem 23, 597–604.
Pilmé, J., Robinson E.A., Gillespie, R.J. 2006. Topological study of the geometry of AF6E molecules: Weak and inactive lone pairs. Inorg Chem 45, 6198–6204.
Pilmé, J., Piquemal, J.-P. 2008. Advancing beyond Charge Analysis using the Electronic Localization Function: Chemically Intuitive Distribution of Electrostatic Moments. J Comput Chem 29, 1440–1449.
Piquemal, J.-P., Pilmé, J. 2006. Comments on the nature of the bonding in oxygenated dinuclear copper enzymes models. J Mol Struct: THEOCHEM 764, 77–86.
Piquemal, J.-P., Chelli, R., Procacci, P., Gresh, N. 2007. Key role of the polarization anisotropy of water in modeling classical polarizable force fields. J Phys Chem A 111, 8170–8176.
Piquemal, J.-P., Pilmé, J., Parisel, O., Gérard, H, Fourré, I., Bergès, J., Gourlaouen, C., de la Lande, A., van Severen, M. C., Silvi, B. 2008. What can be learnt on biological or biomimetic systems with the topological analysis of the electron localization function? Int J Quant Chem 108, 1951–1969.
Rassolov, V., Pople, J.A., Ratner, M., Windus, T.L 1998. 6-31G* basis set for atoms K through Zn. J Chem Phys 109, 1223–1229.
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S., Windus, T.L., Dupuis, M., Montgomery Jr, J.A 1993. General atomic and molecular electronic structure system. J Comput Chem 14, 1347–1363.
Silvi, B., Savin, A 1994. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371, 683–686.
Simonson, T., Claimet, N. 2002. CysxHisy-Zn2+ interactions: Thiol vs. thiolate coordination. Proteins: Structure, Function, and Genetics 49, 37–48.
Stevens, W.J., Basch, H., Krauss, M 1984. Compact erective potentials and efficient shared-exponent basis sets for the first- and second-row atoms. J Chem Phys 81, 6026–6033.
Stevens, W.J., Fink, W.H 1987. Frozen Fragment Reduced Variational Space Analysis of Hydrogen Bonding Interactions. Application to Water Dimer. Chem Phys Lett 139, 15–22.
Zaric, S, Popovic, D.M., Knapp, E.W. 2000. Metal Ligand Aromatic Cation-π Interactions in Metalloproteins: Ligands Coordinated to Metal Interact with Aromatic Residues. Chem Eur J 6, 3935–3942.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
De Courcy, B., Gresh, N. & Piquemal, JP. Importance of lone pair interactions/redistribution in hard and soft ligands within the active site of alcohol dehydrogenase Zn-metalloenzyme: Insights from electron localization function. Interdiscip Sci Comput Life Sci 1, 55–60 (2009). https://doi.org/10.1007/s12539-008-0027-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-008-0027-0