Abstract
Introduction
The endoscopic laser balloon ablation system (EAS) is a relatively novel technique to perform pulmonary vein isolation (PVI) in the treatment of atrial fibrillation (AF). The present study aimed to report the results of the first 50 patients treated in the Netherlands with the EAS in terms of procedural characteristics and AF-free survival.
Methods
Fifty patients successfully underwent EAS PVI. Median follow-up was 17 months. Mean age was 56 years, 82 % had paroxysmal AF.
Results
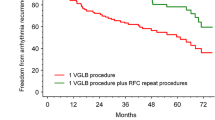
99 % of the pulmonary veins were successfully isolated with the EAS. Mean procedure time was 171 min and mean fluoroscopy time was 36 min. One procedure was complicated by a temporary phrenic nerve palsy (2 %). During follow-up, 58 % of patients remained free of AF without the use of antiarrhythmic drugs.
Conclusion
PVI with EAS is associated with a low risk of complications and a medium-term AF-free survival comparable with other PVI techniques.
Similar content being viewed by others
Introduction
Pulmonary vein isolation (PVI) has become an important treatment modality for atrial fibrillation (AF) [1, 2] although AF recurrences can occur [3]. Several PVI techniques have been developed [4, 5] in an attempt to increase AF-free survival, among which the endoscopic laser balloon ablation system (EAS) [6, 7]. The EAS consists of a flexible, compliant balloon for sustained wall contact and an adjustable laser beam for ablation independent of tissue contact. The present study aims to report the procedural characteristics and AF-free survival after EAS PVI of the first 50 patients treated with the EAS in the Netherlands.
Methods
Fifty consecutive patients who underwent a primo PVI using the EAS in our centre between December 2011 and December 2013 were included in a prospective registry. The prospective registry has been approved by the Institutional Review Board and all patients consented to their data being registered.
Preprocedural care
All patients underwent transoesophageal echocardiography to rule out left atrial (LA) thrombus prior to the procedure. Patients stopped using anticoagulants and were ‘bridged’ with low-molecular-weight heparin until the day of ablation, in accordance with local guidelines.
The endoscopic laser balloon
The EAS (CardioFocus, Marlborough, MA, USA) is a balloon-based catheter system. Its characteristics have been described previously [7, 8]. The EAS was manoeuvred to each pulmonary vein (PV) ostium under fluoroscopic guidance (Fig. 1). A ring of atrial myocardium antral to the PV was exposed by varying the EAS balloon inflation size. Laser energy was delivered to the exposed ring of atrial tissue. After a full circle was completed, the EAS was retracted from the PV. The ablation procedure is also illustrated in an online movie (online movie). A circular mapping catheter was introduced to assess persistent electrical connection between the PV and the left atrium. If any existed, the EAS was re-introduced to the PV, and additional lesions were applied by the operator. No adenosine testing was performed. An oesophageal temperature probe (SensiTherm, St Jude Medical, USA) was inserted, and energy delivery was instantaneously terminated when the temperature exceeded 39.0 °C. During ablation of the right-sided PVs, stimulation of the phrenic nerve (using 20 mA at 2.9 ms) was performed, with immediate cessation of energy delivery once capture was diminished or lost.
Follow-up
Patients visited the outpatient clinic at 3, 6, 12, 18 and 24 months after PVI, including 24-h Holter ECG. AF recurrence was defined in accordance with European guidelines [1]. In all patients, antiarrhythmic drugs were ceased 3 months after the PVI.
Study endpoints
The primary endpoint of our study was AF-free survival after EAS PVI. Secondary endpoints were: acute PVI, procedure time, ablation time and fluoroscopy time. The safety endpoint was major or minor complications within 30 days of the procedure as described in European guidelines [1].
Statistical analysis
Continuous variables were expressed as mean with standard deviation in case of normal distribution or median with interquartile range when not normally distributed. Statistical analysis was performed using IBM SPSS statistics version 20 (IBM inc., Armonk, NY, USA).
Results
Baseline characteristics of the 50 consecutive patients are displayed in Table 1. Mean age was 56 years, 82 % had paroxysmal AF. No LA thrombi were found during the preoperative transoesophageal echocardiogram. There were 2 left-sided and 1 right-sided common PVs and there were 2 right-sided accessory PVs.
Ablation results
In 198 out of 199 PVs (99.5 %), acute PVI was achieved. One common left-sided PV could not be isolated due to a temperature rise of the oesophagus. One procedure was converted to radiofrequency catheter ablation after ablation of the right upper PV was complicated by a phrenic nerve palsy, which did not prolong hospital stay and had fully recovered after 6 months. This was the only complication we observed (2 %). Table 2 displays the procedural characteristics of all patients.
Follow-up
After a median follow-up of 17.3 (interquartile range: 12.9–19.5) months, 58 % of patients were free of AF after a single EAS PVI without the use of antiarrhythmic drugs.
Discussion
The present study reports the results of the first 50 patients treated with the EAS in the Netherlands. Acute PVI can be achieved virtually always, with a low risk of complications. Moreover, medium-term AF-free survival seems to be comparable with other PVI techniques.
The EAS combines a compliant balloon design, an endoscope for visualisation of PV antral tissue and a power-adjustable laser beam. Previous reports have shown that acute PVI can be achieved virtually always with the EAS, which was also observed in the present study [8, 9]. In the present study, the complication rate is low (2 %), which is consistent with previous reports [7, 9].
Although PVI is an important treatment modality for AF, the medium-term AF-free survival is still 60–80 %, after PVI using different techniques, such as radiofrequency catheter ablation [3] and cryoballoon ablation [4]. In the present study, AF-free survival after a single EAS PVI attempt was 58 %, which is in line with a previous report [9], although there are other EAS studies reporting a higher AF-free survival [10, 11]. Potentially, a learning curve may, in part, have affected the AF-free survival in the current study. A randomised trial will provide further evidence of the AF-free survival after EAS [12]. Based on the current literature, the AF-free survival rate after EAS PVI seems to be comparable with other techniques.
AF recurrences are generally regarded as recurrence of electrical conduction over the PV-LA junction [13, 14]. Durable lesion sets therefore remain pivotal in improving medium-term AF-free survival after PVI. The medium-term success percentage suggests the lesion sets created with the EAS are not persistent. This was also suggested in another study [10], which reported that 62 % of the studies patients had 4 isolated PVs 3 months after the initial EAS PVI procedure.
Future studies should be aimed at identifying factors that are associated with AF-free survival after EAS PVI. Although the EAS consists of a compliant balloon, the catheter-tissue contact may be suboptimal in some patients, limiting the operator’s ability to deliver circular, transmural lesion sets which may influence AF-free survival. Moreover, in case of insufficient occlusion or ablation near to a blood pool, ablation energy had to be reduced. One study [15] showed high-energy EAS ablation was favourable to low-energy EAS ablation in terms of persistent electrical conduction over the PV-LA junction and AF-free survival after PVI. Although highly speculative, these factors may explain the medium-term AF-free survival in the present study.
Conclusion
The EAS is a promising technique with a high acute PVI success rate and a low risk of complications. Medium-term AF-free survival after EAS PVI is comparable with other PVI techniques.
References
Calkins H, Kuck KH, Cappato R, et al. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606.
Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New Engl J Med. 1998;339:659–66.
Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–61.
Van Belle Y, Janse P, Rivero-Ayerza MJ, et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28:2231–7.
Yokoyama K, Nakagawa H, Shah DC, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–62.
Reddy VY, Neuzil P, d’Avila A, et al. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm. 2008;5:353–60.
Reddy VY, Neuzil P, Themistoclakis S, et al. Visually-guided balloon catheter ablation of atrial fibrillation: experimental feasibility and first-in-human multicenter clinical outcome. Circulation. 2009;120:12–20.
Dukkipati SR, Neuzil P, Skoda J, et al. Visual balloon-guided point-by-point ablation: reliable, reproducible, and persistent pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2010;3:266–73.
Dukkipati SR, Kuck KH, Neuzil P, et al. Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience. Circ Arrhythm Electrophysiol. 2013;6:467–72.
Dukkipati SR, Neuzil P, Kautzner J, et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: multicenter results of pulmonary vein remapping studies. Heart Rhythm. 2012;9:919–25.
Schmidt B, Metzner A, Chun KR, et al. Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol. 2010;3:481–8.
Catheter Ablation of Drug-refractory Persistent Atrial Fibrillation With the HeartLight(TM) Laser Balloon in Comparison With Irrigated Radiofrequency Current Ablation. http://clinicaltrials.gov/show/NCT01863472. Accessed August 13th, 2014.
Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–604.
Lemola K, Hall B, Cheung P, et al. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004;1:197–202.
Bordignon S, Chun KR, Gunawardene M, et al. Energy titration strategies with the endoscopic ablation system: lessons from the high-dose vs. low-dose laser ablation study. Europace. 2013;15:685–9.
Funding
None
Conflict of interest
None declared
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
(AVI 20782 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gal, P., Smit, J.J.J., Adiyaman, A. et al. First Dutch experience with the endoscopic laser balloon ablation system for the treatment of atrial fibrillation. Neth Heart J 23, 96–99 (2015). https://doi.org/10.1007/s12471-014-0624-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-014-0624-y