Abstract
Purpose of Review
Atrial fibrillation (AF) is the most common arrhythmia in adults. The number of patients with AF is anticipated to increase annually, mainly due to the aging population alongside improved arrhythmia detection. AF is associated with a significantly elevated risk of hospitalization, stroke, thromboembolism, heart failure, and all-cause mortality. Echocardiography is one of the key components of routine assessment and management of AF. Therefore, the aim of this review is to briefly summarize current knowledge on “novel” echocardiographic parameters that may be of value in the management of AF patients.
Recent Findings
Novel echocardiographic biomarkers and their clinical application related to the management of AF have been taken into consideration. Both standard parameters such as atrial size and volume but also novels like atrial strain and tissue Doppler techniques have been analyzed.
Summary
A number of novel echocardiographic parameters have been proven to enable early detection of left atrial dysfunction along with increased diagnosis accuracy. This concerns particularly experienced echocardiographers. Hence, these techniques might improve the prediction of stroke and thromboembolic events among AF patients and need to be further developed and disseminated. Nonetheless, even the standard imaging parameters could be of significant value and should not be discontinued in everyday clinical practice.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in adults. The predicted prevalence of AF by 2030 is more than 15 million Europeans [1]. The incidence of AF increases with age, reaching over 15% by the age of 80, especially in the presence of various cardiovascular risk factors [2]. In the last 2 decades, over 60% increase in AF-related hospitalization has been observed. AF is also associated with progression of diastolic and systolic heart failure, impaired quality of life, and increased all-cause mortality [3,4,5]. Importantly, AF may not cause evident symptoms which makes both diagnosis and treatment a challenge. Indeed, asymptomatic AF has been shown to be one of the reasons for transient ischemic attacks (TIA) or cryptogenic ischemic strokes [6]. Consequently, better understanding of AF, particularly detection of AF seems to be of utmost importance to reduce the number of AF-related complications, particularly stroke [7, 8]. There is also an increasing focus on an integrated or holistic approach to AF management, which has been associated with improved outcomes [9, 10].
The underlying mechanisms of AF are still not fully explained, but may include heterogeneous factors leading to electrical, structural, and mechanical remodelling of the left atrium (LA) [11]. Despite continuous efforts to re-establish sinus rhythm, either with drugs or AF ablation, the only well-proven method to improve prognosis in AF patients remains systemic anticoagulation, which reduces the risk of stroke and all-cause mortality [12]. In addition, there is often no correlation between arrhythmia onset and stroke which further complicates our understanding of AF pathophysiology [13].
Consequently, there has been a greater focus on new diagnostic imaging techniques and new parameters to help better prediction, detection, and treatment of AF in clinical practice. There are multiple imaging modalities and increasing opportunities with cardiac investigations, such as echocardiography. Indeed, echocardiography is becoming an essential component of endovascular invasive procedures such as ablation, left atrial appendage closure, or mitral valve regurgitation treatment [14].
The aim of this review article is to provide an overview of “novel” echocardiography parameters which may be of value in the diagnostic workup and management of patients with AF. We put emphasis on clinically useful parameters and techniques which are gaining in popularity, with the potential for improving patient management.
Atrial Size and Volume
The incidence of AF is associated with structural remodelling and dilatation of LA [15, 16]. It is less clear whether AF is a consequence of LA remodelling and dilatation or vice versa, though both mechanisms are feasible. The precise underlying pathophysiology of LA remodelling is also not entirely clear but include local and systemic inflammation, atrial fibrosis, atrial enlargement, altered autonomic tone, and anatomical and electrical changes [17, 18]. Such structural changes and fibrosis increase the surface for multiple wave propagation, causing AF both to emerge and become more persistent [19].
Atrial fibrosis has been shown in both lone AF and AF secondary to mitral valve diseases. Fibrillar collagen deposits substitute, as a repair process, degenerating myocardial cells causing interstitial expansion and thus deteriorate atrial electrical and mechanical function [20]. LA fibrosis has been shown by delay-enhanced magnetic resonance imaging (DE-MRI) [21, 22]. For example, the Utah scale describes the degree of LA fibrosis (Utah I: minimal fibrosis; Utah IV: extensive fibrosis), which is of clinical importance because the degree of fibrosis may impact on the efficacy of AF ablation [23, 24]. Indeed, observed AF recurrences were more frequent in patients with more advanced LA fibrosis (75%) compared with those with minimal enhancement in DE-MRI (14%) [22]. Furthermore, a significant correlation has been shown recently between LA diastolic function obtained in speckle tracking echocardiography and low amplitude potential area measured with electroanatomical mapping. These findings pointed to use speckle tracking echocardiography as a valuable and useful method in the assessment of LA fibrosis [25]. Consequently, the assessment of LA fibrosis has been proposed as part of the workup for patients undergoing AF ablation.
Patients with recurrent AF have essentially greater left atrial diameters compared with those with sinus rhythm. Indeed, LA enlargement seems to be the key determinant of successful rhythm control in patients with AF. For example, a meta-analysis of 3700 patients [26] and other reports [27,28,29] clearly show that a dilated LA significantly increases the risk for AF recurrence. External cardioversion and catheter ablation in AF patients with LA diameter of 50–55 mm are of limited value [30]. LA diameter as assessed by transthoracic echocardiography in parasternal long axis view (PLAX) with a diameter of > 43 mm was the cutoff value for prediction of paroxysmal AF recurrence after catheter ablation [31]. The AFFIRM study also showed that recurrent AF occurs more often with larger LA diameter, with a hazard ratio (HR) for AF relapse of 1.21 for LA diameter of 4.1–4.5 cm and 1.32 for LA > 5.5 cm [32].
Despite the fact that LA diameter is commonly measured in clinical practice, its predictive value might be limited compared with the assessment of the LA Volume Index (LAVI). This is mainly driven by possible misleading in antero-posterior linear dimension due to distorted atrium. Furthermore, LAVI was shown to be more strongly associated with cardiovascular diseases compared with the linear dimension [33, 34]. The latter could be obtained using both two- and three-dimensional echocardiography [35, 36]. Importantly, assessment of LAVI combined with various biomarkers (D-dimer, prothrombin, thrombin-antithrombin complex, and fibrin monomer) may be of value in identifying patients with underlying (but undetected) AF and cryptogenic stroke [37]. LAVI was also shown to be a predictor of AF recurrence after mitral valve (MV) surgery and successful external cardioversion. LAVI > 39 mL/m2 was an independent predictor for AF after MV surgery [38]. For those AF patients who were externally cardioverted, LAVI showed higher predictive value for AF recurrence compared with a simple assessment of LA diameter (AUC 0.78 vs 0.56 respectively, p = 0.003) [39]. Dimensions of the right atrium (RA) may also be of clinical importance in patients with AF. Increased RA volume index (RAVI) may affect the efficacy of catheter ablation. Indeed, it has been shown that in patients being 3 months after the ablation RAVI > 78 mL/m2 predicted AF relapse with 74% sensitivity and 68% specificity [40].
Left Atrial Strain
The term “strain” means deformation of the myocardium and is used to describe the mechanical function of the atrium or ventricle throughout the cardiac cycle. In the atria, reservoir, conduit, and booster function can be assessed. The maximum myocardial shortening of LA during atrial systole is defined as the peak atrial contraction strain (PACS) and the velocity of this deformation is termed the “strain rate.” Maximal deformation of the LA myocardium during systole of the left ventricle has been described as peak atrial longitudinal strain (PALS) and reflects the reservoir function of the chamber. For echocardiographic measurements in AF, similar RR intervals and heart rate of < 100/min are important, whereas the number of averaged cardiac cycles is of less value [41].
Left Peak Atrial Longitudinal Strain
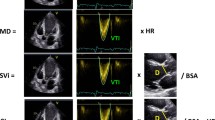
Left peak atrial longitudinal strain (PALS) is perceived as a parameter of atrial distension that immediately reflects any changes in heart rhythm [42]. In one prospective study, PALS (see Fig. 1) was measured before and soon after cardioversion. The parameter increased immediately following the restoration of sinus rhythm by electrical cardioversion (11.9 ± 1 vs 15.9 ± 1.3 respectively). In addition, among patients with a higher increase in PALS, the incidence of AF recurrence was lower over a 6-month observation; indeed, the difference in strain, obtained by regression analysis adjusted for age, sex, and body mass index (BMI), was shown as a predictor of sinus rhythm maintenance [42]. Several studies have showed that PALS may be of value in predicting successful AF catheter ablation (see Table 1) [43,44,45,46].
a, b Representative strain curve of LA with R-R gating. The functional phases of LA mechanics are shown in sinus rhythm (1A) and atrial fibrillation (1B). In atrial fibrillation peak, the atrial contractile strain has disappeared. Dotted line means averaged peak LA strain. PALS, peak atrial longitudinal strain; PACS, peak atrial contractile strain
Conversely, reduced PALS is associated with a higher risk of secondary AF (after MV surgery or CABG [47,48,49,50]). Importantly, lower PALS is linked not only with AF development or recurrence but also with the higher risk for stroke in paroxysmal, permanent, and persistent AF [46, 51]. PALS is predictive of the risk of AF in patients with cryptogenic stroke [52].
LA Dispersion
Dispersion is defined as a loss of coordinated myocardial contraction (dyssynchrony). The index of LA dispersion has been proposed to accurately measure the extent of dyssynchrony and potential impact on LA function, probability of arrhythmia (in particular AF) and prognosis (e.g., risk of stroke). The index of LA dispersion has been defined as standard deviation (SD) of the time to the PALS in 12 LA segments, projected in the 2 apical views (see Fig. 2), which correlates with LA remodeling and brain natriuretic peptide (BNP) concentrations [53]. A negative correlation between the index of LA dispersion and PALS (r = − 0.44) may suggest that the contractility of the LA is lower in a dyssynchronized LA [53]. On multivariate analysis, adjusted for the pre-cardioversion clinical and echocardiographic variables, the index of LA dispersion was an independent predictor of 1-year AF recurrence following cardioversion [54]. Indeed, dispersion of time to PALS may be of value in the prediction of sinus rhythm maintenance. The parameter lower than a cut-off point of 128 ms predicted sinus rhythm at 6 months after CV with 66% accuracy [55]. Furthermore, LA mechanical dispersion, defined as SD of contraction times of 18 LA segments, was reported to predict AF recurrence after catheter ablation (cutoff value of 24 ms, sensitivity of 77%, and a specificity of 85%, AUC 0.88) [56]. Thus, LA dispersion is in different works assessed as SD of 12 or 18 segments contraction durations or the maximal difference of contraction durations in 12 atrial segments.
The example of temporal analysis of LA contraction—two-dimensional speckle-tracking based LA strain curves derived from a four-chamber apical view. Each curve represents one of the six LA wall segments; dotted line means averaged peak LA strain. White arrows indicate time to peak atrial longitudinal strain from each of the visualized 6 segments. Indices derived from the time to peak strain of each segment are considered in dyssynchrony (dispersion) analysis. Usually, the standard deviation of time to peak strain is taken (see chapter: LA dispersion)
The electrical response to cardioversion (namely sinus rhythm) may not be consistent with endocrine and mechanical remodelling responses of the heart, at least in the early phase (e.g., 1 month after cardioversion). For example, patients with sinus rhythm 1 month after cardioversion had similar pro-atrial natriuretic peptide (proANP) levels and PALS compared with those with persistent AF, even though sinus rhythm restoration was associated with lowering of the LAVI; thus, alternations in LA volume may precede changes in LA remodeling [57].
LA Stiffness
LA stiffness, defined as the ratio of delta pressure and volume in the left ventricle (LV), can be easily computed when pressure/volume curves are obtained during invasive procedure, e.g., pulmonary veins isolation. LV longitudinal strain has been identified as the only independent predictor of LA stiffness, and the addition of a LV stiffness parameter (based on MV deceleration time [58]) to the LA stiffness predictor increases sensitivity and specificity of AF recurrence prediction up to 72% and 75%, respectively [53]. Because systolic parameters in AF patients are not easily reproducible and diastolic measures are better validated against invasive measurements, LA strain may have promising use clinically [41].
Two-dimensional speckle-tracking may also be valuable in predicting paroxysmal AF, especially in patients with ischemic stroke or TIA [59]. Early diastolic strain rate (SRe) and global longitudinal displacement (GLD) of the left ventricle were significantly lower in patients with paroxysmal AF compared with those without arrhythmia [6]. These parameters were retrospectively assessed in 205 patients who survived acute cerebrovascular ischemia (cryptogenic stroke or TIA), were in sinus rhythm, and underwent echocardiography examination: none of the conventional echocardiographic parameters but lower GLD and lower SRe were significantly associated with paroxysmal AF [6].
Due to its high sensitivity in determining subtle abnormalities, two-dimensional speckle-tracking can provide an early assessment of left ventricular (LV) diastolic dysfunction given there is an association with reduced reservoir, conduit, and LA contraction. Therefore, the diagnosis can be established sooner than with the use of standard echocardiography measurements [60]. Moreover, in impaired LA deformation visualized by significant reduction of longitudinal systolic strain rate (SRs), systolic strain (SR) and SRe have been independently associated with the presence of left atrial appendage (LAA) thrombus; hence, speckle-tracking might provide an additional, clinically practical tool in identifying patients at risk for LAA thrombus formation [61].
Total Conduction Time
The stage of atrial fibrosis correlates with AF-burden, defined as the percentage of time spent in arrhythmia [62,63,64]. This degree of atrial fibrosis can be estimated by total atrial conduction time (PA-TDI) which is the time interval between the onset of P-wave in lead II of the ECG on echocardiographic images to the peak A´-wave of the lateral atrial wall on the tissue Doppler tracing. One analysis that assessed the relationship between LA reservoir strain and PA-TDI in AF patients, as compared with those without AF, showed that patients with paroxysmal and persistent AF had significantly longer PA-TDI and a progressive decline in LA reservoir [65]. LA reservoir function correlated negatively with PA-TDI which may suggest that the burden of LA fibrosis and LA structural remodelling affects the progression of AF [65].
PA-TDI can also be useful in the prediction of new-onset AF after acute myocardial infarction. LA maximal volume (HR 1.07), total LA ejection fraction (HR 0.96), and PA-TDI duration (HR 1.05) were independent predictors of new-onset AF in patients after acute myocardial infarction [66]. Moreover, PA-TDI > 145 ms (93.8% sensitivity and 90.5% specificity) was shown to predict AF in patients with cryptogenic stroke [67]. Also, intra (left) atrial mechanical delay obtained with simultaneous recording of Doppler waveforms at two different sites (tissue Doppler echocardiography) may predict AF recurrence [68].
Diastolic and Systolic Tissue Doppler Parameters
Simple and objective parameters defining the risk of arrhythmia recurrence would be helpful in tailoring AF treatment. Ari et al. investigated the predictive value of pre-cardioversion tissue Doppler parameters which link systolic atrial (Aa) and systolic ventricular (Sa) functions with diastolic atrial function—LV filling pressure (E/Ea). Preliminary results, based on 127 patients showed that the indices were significantly higher in the AF recurrence group compared with the sinus rhythm group [69].
LA Appendage
In the healthy heart, the LA appendage (LAA) is a location for reduced contractility and stasis within the LA. Thus, in specific circumstances (e.g., in patients with AF), it is often the location for thrombus formation. Indeed, approximately 90% of AF-related thrombi are localized in LAA. The shape of the LAA can be classified into four groups: wind-sock, chicken-wing, cauliflower, cactus-like (see Fig. 5) [70]. Compared with non-chicken morphology, the chicken-wing morphology of LAA was found to be associated with significantly decreased thromboembolic risk in AF. Conversely, compared with chicken-wing, cactus and windsock were associated with a 4-fold and cauliflower with an 8-fold increase in stroke or TIA risk [71].
LAA Contraction
Evaluation of LAA contraction is crucial to assess the potential for thrombus formation. Under normal circumstances, that is in sinus rhythm and when contractility of the LA is preserved, the apex of LAA almost obliterates. The emptying velocity, that is highest in the proximal part of LAA, ranges from 50 to 83 cm/s [72]. Velocities < 40 cm/s are considered as increasing the risk for stroke [73]. Of note, LAA emptying velocity should be measured at the LAA orifice up to 2 mm inside LAA due to its dependency on the measurement depth [74]. Velocities < 20 cm/s (usually with the presence of spontaneous echo contrast) have been associated with high thromboembolic risk (see Fig. 3) [73].
Even in low CHADS2 score patients (≤ 1) LA emptying velocity and LAA ejection fraction (LAAEF) were valuable predictors of thrombus formation. For LAAEF measurement, both disk summation method and endocardial border speckle tracking (e.g., by velocity vector imaging) can be successfully utilized [75, 76]. For thrombus prediction, AAEF of 21% (as the cutoff value) showed 93% sensitivity and 96% specificity, whereas the corresponding values for LAA emptying velocity (cutoff value of 24 cm/sec) were 73% and 75% (AUC 0.73), respectively [75].
Tissue velocity imaging has been used to assess the LAA walls velocities (both in transthoracic (TTE) and transesophageal echocardiography (TEE)) and the risk of thrombus formation [77]. Severe spontaneous echo contrast in LAA was found with 72% sensitivity and 82% specificity if TDI mean velocity was < 6.13 cm/s. On regression analysis, LAA tissue Doppler velocity was the only predictor of qualitative LAA spontaneous contrast grade [78]. Moreover, LAA wall velocity assessed by spectral tissue Doppler can predict sinus rhythm maintenance after catheter ablation with 60% sensitivity and 91% specificity [43]
The higher the emptying velocity before CV is, the higher the probability of sinus rhythm 1 year after CV is, with the velocity exceeding 40 cm/s having 56% sensitivity and 80% specificity [28]. Conversion of AF to sinus rhythm is often accompanied by transient mechanical dysfunction of LAA, called atrial stunning. Stunning is defined as LAA peak diastolic emptying velocity lower than 20 cm/s [70]. Such stunning after successful cardioversion may last from hours to weeks—this is why anticoagulation is indicated for four weeks afterward.
LAA in 3 Dimensions
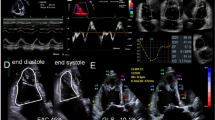
Three-dimensional (3D) echocardiography seems to be very helpful in LAA shape and detailed morphology assessment. 3D TEE is superior to 2D TEE in differentiating pectinate muscles, thrombus, myocardium or calcification, lysis, and structure mobility (see Fig. 4) [79]. Moreover, volumes or LAA orifices are likely to be less underestimated in 3D compared with 2D, and 3D tools can be utilized in automated volume calculation [80,81,82]. LAA orifice is measured due to easily obtainable views in 3D TEE [70, 80, 83]
Conclusions: Keeping It Simple
There are several “novel” echocardiographic parameters that hold promise to be more accurate in the assessment of both LA function and very early LA dysfunction and thus they may further improve prediction of AF, stroke, heart failure, or death compared with standard echocardiographic indices. For now, these parameters have not been included in standard echocardiography examination because they require an experienced echocardiographer, have limited reproducibility, prolong the time of examination, but most importantly have not been shown to be substantially superior to very simple and easy to use parameters that can be measured by everybody in routine clinical practice.
The best example of such an “easy” parameter is LA size (perhaps also LA volume), and regardless of limitations associated with a simple measurement of this parameter, it may have robust clinical significance. Indeed, echocardiography may become a crucial diagnostic tool to guide therapy in some niche areas of clinical practice. For example, it may predict AF, stroke, or both in patients with a history of cryptogenic stroke and no evidence of AF. Two recent trials (NAVIGATE ESUS and RE-SPECT ESUS) failed to show the benefit of therapy with rivaroxaban and dabigatran respectively versus aspirin for the prevention of recurrent strokes in patients with the history of cryptogenic stroke [84, 85]. However, subanalysis of the NAVIGATE ESUS trial showed that in patients with LA diameter of more than 4.6 cm (the risk factor for AF development) the risk of stroke at 1 year was significantly lower for therapy with rivaroxaban (1.7%) than with aspirin (6.5%) (HR 0.26; 95% CI, 0.07–0.94) [86].
Another niche area for echocardiography may be improved stroke risk stratification in patients with AF and truly low risk of stroke, that is CHA2DS2-VASc 0 in men and 1 in women. For now, there is no clear evidence for the positive net clinical benefit of oral anticoagulation (when balancing the risk of stroke versus the risk of major bleeding, particularly intracranial hemorrhage) in such patients. However, “low-risk” is not homogenous and static and there may be some patients who would benefit from anticoagulation. Indeed, one recent report shows that oral anticoagulation may be beneficial in very young AF patients, e.g., those aged 35 or more with concomitant heart failure (CHA2DS2-VASc = 1) [87]. Assessment of LA size (see Fig. 5), volume, LAA morphology (e.g., non-chicken morphology) and perhaps also more sophisticated indices (e.g., PALS) may help stratify stroke risk and guide anticoagulation in AF patients presently perceived as being at a very low risk of stroke.
References:
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. https://doi.org/10.2147/CLEP.S47385.
Allan V, Honarbakhsh S, Casas J-P, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? Thromb Haemost. 2017;117(05):837–50. https://doi.org/10.1160/TH16-11-0825.
Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol. 2002;13(4):399-405. http://www.ncbi.nlm.nih.gov/pubmed/12033360. Accessed June 10, 2019.
Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality. Circulation. 2003;107(23):2920–5. https://doi.org/10.1161/01.CIR.0000072767.89944.6E.
Darby AE. Management Of atrial fibrillation in patients with heart failure. J Atr Fibrillation. 2014;7(2):1105. https://doi.org/10.4022/jafib.1105.
Skaarup KG, Christensen H, Høst N, et al. Usefulness of left ventricular speckle tracking echocardiography and novel measures of left atrial structure and function in diagnosing paroxysmal atrial fibrillation in ischemic stroke and transient ischemic attack patients. Int J Cardiovasc Imaging. 2017;33(12):1921–9. https://doi.org/10.1007/s10554-017-1204-1.
Lip GYH, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Thromb Haemost. 2017;117(07):1230–9. https://doi.org/10.1160/TH16-11-0876.
Lip GYH, Banerjee, Amitava, Boriani G, et al. Antithrombotic therapy for atrial fibrillation CHEST guideline and expert panel report. 2018. https://doi.org/10.1016/j.chest.2018.07.040.
Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–8. https://doi.org/10.1038/nrcardio.2017.153.
Pastori D, Pignatelli P, Menichelli D, Violi F, Lip GYH. Integrated care management of patients with atrial fibrillation and risk of cardiovascular events. Mayo Clin Proc. December 2018. https://doi.org/10.1016/j.mayocp.2018.10.022.
Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med. 2009;20(7):672–81. https://doi.org/10.1016/j.ejim.2009.07.011.
Lip GYH, Fauchier L, Freedman SB, et al. Atrial fibrillation. Nat Rev Dis Prim. 2016;2(1):16016. https://doi.org/10.1038/nrdp.2016.16.
Damluji AA, Al-Damluji MS, Marzouka GR, et al. New-onset versus prior history of atrial fibrillation: outcomes from the AFFIRM trial. Am Heart J. 2015;170(1):156-163.e1. https://doi.org/10.1016/j.ahj.2015.04.020.
Kim T-S, Youn H-J. Role of echocardiography in atrial fibrillation. J Cardiovasc Ultrasound. 2011;19(2):51–61. https://doi.org/10.4250/jcu.2011.19.2.51.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. https://doi.org/10.1093/eurheartj/ehw210.
Corradi D, Callegari S, Maestri R, Benussi S, Alfieri O. Structural remodeling in atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5(12):782–96. https://doi.org/10.1038/ncpcardio1370.
Waldo AL. Mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14(12 Suppl):S267-S274. http://www.ncbi.nlm.nih.gov/pubmed/15005213. Accessed June 10, 2019.
Weiss JN, Qu Z, Chen P-S, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112(8):1232–40. https://doi.org/10.1161/CIRCULATIONAHA.104.529545.
Liżewska-Springer A, Dąbrowska-Kugacka A, Lewicka E, Drelich Ł, Królak T, Raczak G. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: a literature review. Cardiol J. 2013. https://doi.org/10.5603/CJ.a2018.0067.
Burstein B, Nattel S. Atrial Fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9. https://doi.org/10.1016/J.JACC.2007.09.064.
Daccarett M, Badger TJ, Akoum N, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(7):831–8. https://doi.org/10.1016/j.jacc.2010.09.049.
Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–67. https://doi.org/10.1161/CIRCULATIONAHA.108.811877.
Akkaya M, Marrouche N, Higuchi K, et al. The degree of left atrial structural remodeling impacts left ventricular ejection fraction in patients with atrial fibrillation. Turk Kardiyol Dern Ars. 2014;42(1):11–9. https://doi.org/10.5543/tkda.2014.20726.
Chelu MG, King JB, Kholmovski EG, et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J Am Heart Assoc. 2018;7(23):e006313. https://doi.org/10.1161/JAHA.117.006313.
Pilichowska-Paszkiet E, Baran J, Sygitowicz G, et al. Noninvasive assessment of left atrial fibrosis. Correlation between echocardiography, biomarkers, and electroanatomical mapping. Echocardiography. 2018;35(9):1326–34. https://doi.org/10.1111/echo.14043.
Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace. 2012;14(5):638–45. https://doi.org/10.1093/europace/eur364.
PARIKH SS, JONS C, MCNITT S, DAUBERT JP, SCHWARZ KQ, HALL B. Predictive capability of left atrial size measured by CT, TEE, and TTE for recurrence of atrial fibrillation following radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2010;33(5):532–40. https://doi.org/10.1111/j.1540-8159.2010.02693.x.
Antonielli E, Pizzuti A, Pálinkás A, et al. Clinical value of left atrial appendage flow for prediction of long-term sinus rhythm maintenance in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2002;39(9):1443-1449. http://www.ncbi.nlm.nih.gov/pubmed/11985905. Accessed March 3, 2019.
Okçün B, Yigit Z, Küçükoglu MS, et al. Predictors for maintenance of sinus rhythm after cardioversion in patients with nonvalvular atrial fibrillation. Echocardiography. 2002;19(5):351-357. http://www.ncbi.nlm.nih.gov/pubmed/12174197. Accessed March 3, 2019.
Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Hear Rhythm. 2012;9(4):632-696.e21. https://doi.org/10.1016/j.hrthm.2011.12.016.
Y-C LIAO, J-N LIAO, L-W LO, et al. Left atrial size and left ventricular end-systolic dimension predict the progression of paroxysmal atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2017;28(1):23–30. https://doi.org/10.1111/jce.13115.
Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? J Am Coll Cardiol. 2005;45(12):2026–33. https://doi.org/10.1016/j.jacc.2005.03.020.
Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr. 2005;18(12):1440–63. https://doi.org/10.1016/j.echo.2005.10.005.
Mor-Avi V, Yodwut C, Jenkins C, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5(8):769–77. https://doi.org/10.1016/j.jcmg.2012.05.011.
Badano LP, Pezzutto N, Marinigh R, et al. How many patients would be misclassified using M-mode and two-dimensional estimates of left atrial size instead of left atrial volume? A three-dimensional echocardiographic study. J Cardiovasc Med. 2008;9(5):476–84. https://doi.org/10.2459/JCM.0b013e3282f194f0.
Zhang Q, Wang J, Dong Q, et al. Evaluation of left atrial volume and function using single-beat real-time three-dimensional echocardiography in atrial fibrillation patients. BMC Med Imaging. 2017;17(1):44. https://doi.org/10.1186/s12880-017-0215-7.
Ellis D, Rangaraju S, Duncan A, et al. Coagulation markers and echocardiography predict atrial fibrillation, malignancy or recurrent stroke after cryptogenic stroke. Medicine (Baltimore). 2018;97(51):e13830. https://doi.org/10.1097/MD.0000000000013830.
Kang M-K, Joung B, Shim CY, et al. Post-operative left atrial volume index is a predictor of the occurrence of permanent atrial fibrillation after mitral valve surgery in patients who undergo mitral valve surgery. Cardiovasc Ultrasound. 2018;16(1):5. https://doi.org/10.1186/s12947-018-0123-1.
Marchese P, Malavasi V, Rossi L, et al. Indexed left atrial volume is superior to left atrial diameter in predicting nonvalvular atrial fibrillation recurrence after successful cardioversion: a prospective study. Echocardiography. 2012;29(3):276–84. https://doi.org/10.1111/j.1540-8175.2011.01580.x.
Moon J, Hong YJ, Shim J, et al. Right atrial anatomical remodeling affects early outcomes of nonvalvular atrial fibrillation after radiofrequency ablation. Circ J. 2012;76(4):860-867. http://www.ncbi.nlm.nih.gov/pubmed/22293450. Accessed March 3, 2019.
Kotecha D, Mohamed M, Shantsila E, Popescu BA, Steeds RP. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace. 2017;19(9):1427–38. https://doi.org/10.1093/europace/eux027.
Shaikh AY, Maan A, Khan UA, et al. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovasc Ultrasound. 2012;10(1):1. https://doi.org/10.1186/1476-7120-10-48.
Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ J. 2013;77(7):1695–704. https://doi.org/10.1253/circj.cj-12-1518.
Motoki H, Negishi K, Kusunose K, et al. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr. 2014;27(11):1184–92. https://doi.org/10.1016/j.echo.2014.08.017.
Hammerstingl C, Schwekendiek M, Momcilovic D, et al. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol. 2012;23(3):247–55. https://doi.org/10.1111/j.1540-8167.2011.02177.x.
Shih J-Y, Tsai W-C, Huang Y-Y, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24(5):513–9. https://doi.org/10.1016/j.echo.2011.01.016.
Candan O, Ozdemir N, Aung SM, et al. Left atrial longitudinal strain parameters predict postoperative persistent atrial fibrillation following mitral valve surgery: a speckle tracking echocardiography study. Echocardiography. 2013;30(9):n/a-n/a. https://doi.org/10.1111/echo.12222.
Ancona R, Comenale Pinto S, Caso P, et al. Two-dimensional atrial systolic strain imaging predicts atrial fibrillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J Am Soc Echocardiogr. 2013;26(3):270–7. https://doi.org/10.1016/j.echo.2012.11.016.
Gabrielli L, Corbalan R, Córdova S, et al. Left atrial dysfunction is a predictor of postcoronary artery bypass atrial fibrillation: association of left atrial strain and strain rate assessed by speckle tracking. Echocardiography. 2011;28(10):1104–8. https://doi.org/10.1111/j.1540-8175.2011.01518.x.
Her AY, Kim JY, Kim YH, et al. Left atrial strain assessed by speckle tracking imaging is related to new-onset atrial fibrillation after coronary artery bypass grafting. Can J Cardiol. 2013;29(3):377–83. https://doi.org/10.1016/j.cjca.2012.06.006.
Shavarov AA, Yusupov AA, Kiyakbaev GK, Vasyuk YA, Moiseev VS. Left atrial remodeling and thromboembolic risk in patients with recurrent atrial fibrillation. Kardiologiia. 2015;55(11):37-44. http://www.ncbi.nlm.nih.gov/pubmed/27125103. Accessed May 6, 2019.
Pagola J, González-Alujas T, Flores A, et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke. 2014;45(8):164–6. https://doi.org/10.1161/strokeaha.114.005540.
Marino PN, Degiovanni A, Baduena L, et al. Non-invasively estimated left atrial stiffness is associated with short-term recurrence of atrial fibrillation after electrical cardioversion. J Cardiol. 2017;69(5):731–8. https://doi.org/10.1016/j.jjcc.2016.07.013.
Dell’Era G, Rondano E, Franchi E, Marino PN. Atrial asynchrony and function before and after electrical cardioversion for persistent atrial fibrillation. Eur J Echocardiogr. 2010;11(7):577–83. https://doi.org/10.1093/ejechocard/jeq010.
Doruchowska A, Wita K, Bochenek T, et al. Role of left atrial speckle tracking echocardiography in predicting persistent atrial fibrillation electrical cardioversion success and sinus rhythm maintenance at 6 months. Adv Med Sci. 2014;59(1):120–5. https://doi.org/10.1016/j.advms.2013.10.003.
Sarvari SI, Haugaa KH, Stokke TM, et al. Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17(6):660–7. https://doi.org/10.1093/ehjci/jev185.
Bernard-Brunet A, Saint Etienne C, Piver E, et al. Incomplete recovery of mechanical and endocrine left atrial functions one month after electrical cardioversion for persistent atrial fibrillation: a pilot study. J Transl Med. 2014;12(1):2–9. https://doi.org/10.1186/1479-5876-12-51.
Marino P, Little WC, Rossi A, et al. Can left ventricular diastolic stiffness be measured noninvasively? J Am Soc Echocardiogr. 2002;15(9):935-943. http://www.ncbi.nlm.nih.gov/pubmed/12221410. Accessed May 6, 2019.
Fu H, Liu T, Zhou C, Zheng C, Li G. Two-dimensional speckle tracking echocardiography: a novel approach to evaluate left atrial mechanical function. Int J Cardiol. 2012;155(3):481–2. https://doi.org/10.1016/j.ijcard.2011.12.072.
Jarasunas J, Aidietis A, Aidietiene S. Left atrial strain - an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc Ultrasound. 2018;16(1):29. https://doi.org/10.1186/s12947-018-0147-6.
Kupczynska K, Michalski BW, Miskowiec D, et al. Association between left atrial function assessed by speckle-tracking echocardiography and the presence of left atrial appendage thrombus in patients with atrial fibrillation. Anatol J Cardiol. 2017;18(1):15–22. https://doi.org/10.14744/AnatolJCardiol.2017.7613.
Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54(2):230-246. http://www.ncbi.nlm.nih.gov/pubmed/12062329. Accessed March 6, 2019.
Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58(21):2225–32. https://doi.org/10.1016/j.jacc.2011.05.061.
Teh AW, Kistler PM, Lee G, et al. Long-term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Hear Rhythm. 2012;9(4):473–80. https://doi.org/10.1016/j.hrthm.2011.11.013.
Leung M, Abou R, van Rosendael PJ, et al. Relation of echocardiographic markers of left atrial fibrosis to atrial fibrillation burden. Am J Cardiol. 2018;122(4):584–91. https://doi.org/10.1016/j.amjcard.2018.04.047.
Antoni ML, Bertini M, Atary JZ, et al. Predictive value of total atrial conduction time estimated with tissue doppler imaging for the development of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol. 2010;106(2):198–203. https://doi.org/10.1016/j.amjcard.2010.02.030.
Müller P, Ivanov V, Kara K, et al. Total atrial conduction time to predict occult atrial fibrillation after cryptogenic stroke. Clin Res Cardiol. 2017;106(2):113–9. https://doi.org/10.1007/s00392-016-1029-2.
Zhou Y, Chen J, Hu B, Cao S, Zhou Q, Guo R. The predictive value of intra-left atrial mechanical delay for 1-year recurrence of atrial fibrillation after catheter ablation: a clinical follow-up study using dual Doppler echocardiography. J Clin Ultrasound. 2018;46(8):519–26. https://doi.org/10.1002/jcu.22629.
Ari H, Ari S, Sarigül OY, et al. A novel index combining diastolic and systolic tissue doppler parameters for prediction of atrial fibrillation recurrence. Echocardiography. 2016;33(7):1009–15. https://doi.org/10.1111/echo.13212.
Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7(12):1251–65. https://doi.org/10.1016/j.jcmg.2014.08.009.
Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? J Am Coll Cardiol. 2012;60(6):531–8. https://doi.org/10.1016/j.jacc.2012.04.032.
Mikael Kortz RA, Delemarre BJ, van Dantzig JM, Bot H, Kamp O, Visser CA. Left atrial appendage blood flow determined by transesophageal echocardiography in healthy subjects. Am J Cardiol. 1993;71(11):976-981. http://www.ncbi.nlm.nih.gov/pubmed/8465792. Accessed May 6, 2019.
Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23(4):961-969. http://www.ncbi.nlm.nih.gov/pubmed/8106703. Accessed May 6, 2019.
Goldberg YH, Gordon SC, Spevack DM, Gordon GM. Disparities in emptying velocity within the left atrial appendage. Eur J Echocardiogr. 2010;11(3):290–5. https://doi.org/10.1093/ejechocard/jep216.
Ono K, Iwama M, Kawasaki M, et al. Motion of left atrial appendage as a determinant of thrombus formation in patients with a low CHADS2 score receiving warfarin for persistent nonvalvular atrial fibrillation. Cardiovasc Ultrasound. 2012;10(1):1. https://doi.org/10.1186/1476-7120-10-50.
Iwama M, Kawasaki M, Tanaka R, et al. Left atrial appendage emptying fraction assessed by a feature-tracking echocardiographic method is a determinant of thrombus in patients with nonvalvular atrial fibrillation. J Cardiol. 2012;59(3):329–36. https://doi.org/10.1016/j.jjcc.2012.01.002.
Uretsky S, Shah A, Bangalore S, et al. Assessment of left atrial appendage function with transthoracic tissue Doppler echocardiography. Eur J Echocardiogr. 2009;10(3):363–71. https://doi.org/10.1093/ejechocard/jen339.
DONAL E, SALLACH J, DANIELMURRAY R, et al. Contrast-enhanced tissue Doppler imaging of the left atrial appendage is a new quantitative measure of spontaneous echocardiographic contrast in atrial fibrillation. Eur J Echocardiogr. 2006;9(1):5–11. https://doi.org/10.1016/j.euje.2006.10.001.
Anwar AM, Nosir YFM, Ajam A, Chamsi-Pasha H. Central role of real-time three-dimensional echocardiography in the assessment of intracardiac thrombi. Int J Cardiovasc Imaging. 2010;26(5):519–26. https://doi.org/10.1007/s10554-010-9593-4.
Nucifora G, Faletra FF, Regoli F, et al. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography. Circ Cardiovasc Imaging. 2011;4(5):514–23. https://doi.org/10.1161/CIRCIMAGING.111.963892.
Agoston I, Xie T, Tiller FL, Rahman AM, Ahmad M. Assessment of left atrial appendage by live three-dimensional echocardiography: early experience and comparison with transesophageal echocardiography. Echocardiography. 2006;23(2):127–32. https://doi.org/10.1111/j.1540-8175.2006.00181.x.
Perk G, Biner S, Kronzon I, et al. Catheter-based left atrial appendage occlusion procedure: role of echocardiography. Eur Hear J - Cardiovasc Imaging. 2012;13(2):132–8. https://doi.org/10.1093/ejechocard/jer158.
Shah SJ, Bardo DME, Sugeng L, et al. Real-time three-dimensional transesophageal echocardiography of the left atrial appendage: initial experience in the clinical setting. J Am Soc Echocardiogr. 2008;21(12):1362–8. https://doi.org/10.1016/j.echo.2008.09.024.
Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191–201. https://doi.org/10.1056/NEJMoa1802686.
Diener H-C, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906–17. https://doi.org/10.1056/NEJMoa1813959.
Healey JS, Gladstone DJ, Swaminathan B, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 2019. https://doi.org/10.1001/jamaneurol.2019.0617.
Chao T-F, Lip GYH, Lin Y-J, et al. Age threshold for the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehy837.
Acknowledgements
Jakub Gumprecht was supported by the Polish Cardiac Society Club 30 Specialized Research Fellowship Grant for Early Career Researchers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gregory Lip declares he is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo, and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
Michał Mazurek has received consultant fees from Biotronik, Boston Scientific, Medtronic, and St. Jude Medical/Abbott
All other authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Echocardiography
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gumprecht, J., Szulik, M., Domek, M. et al. Novel Echocardiographic Biomarkers in the Management of Atrial Fibrillation. Curr Cardiovasc Imaging Rep 12, 43 (2019). https://doi.org/10.1007/s12410-019-9520-6
Published:
DOI: https://doi.org/10.1007/s12410-019-9520-6