Abstract
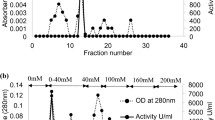
Soluble acid invertases from the fresh and stale cane juice of variety CoJ83 were purified by fractional precipitation with ammonium sulphate and ion exchange chromatography with DEAE- cellulose. Three isoforms of acid invertase were identified in juice of fresh cane and four isoforms of enzyme were found in juice of stale cane. The isoforms obtained were characterized for their kinetic parameters. Optimum pH and optimum temperature range of isoforms from fresh cane juice were 4.5–5.0 and 35–45°C, however isoforms of invertase from juice of stale cane were having optimum pH of 4.0–4.5 and optimum temperature 40–55°C. Isoforms identified from juice of stale canes were kinetically more efficient in comparison to isoforms identified from juice of fresh canes, as they were having higher V max /K m values than isoforms from fresh cane juice. MnCl2 inhibited the soluble acid invertase isoforms of fresh cane completely, but it was unable to inhibit completely the enzyme isoforms of juice of stale cane. ZnCl2, NiCl2, KCl, sodium metasilicate, sodium lauryl sulphate and KMnO4 inhibited the activity of different invertase isoforms in both fresh and stale cane juice, but this inhibition percent was relatively less in stale cane enzyme isoforms as compared to enzyme isoforms from juice of fresh cane. This points to the existence of structural and conformational differences among invertase isoforms in juice of fresh and stale cane.




Similar content being viewed by others
References
Alexander, A.G. 1973. A comprehensive study of Saccharum source to sink system. Sugarcane Physiology, 472. Amsterdam: Elsevier Scientific Pub Comp.
Batta, S.K., J. Singh, K.P. Sharma, and R. Singh. 1991. Kinetic properties and inhibition of soluble acid invertase from sugarcane juice. Plant Physiology and Biochemistry 29: 415–419.
Batta, S.K., and R. Singh. 1991. Post harvest deterioration in quality of sugarcane. bharatiya Sugar 32: 49–51.
Batta, S.K., B. Kaur, J.S. Sital, S.K. Sandhu, and S.K. Uppal. 2011. Sucrose accumulation and internodal soluble invertase isoenzymes in plant and ratoon crops of sugarcane. Sugar Tech 13: 51–59.
Batta, S.K., Benkush, J.S. Sital, and A.P.S. Mann. 2006. Kinetic and thermodynamic properties of internodal soluble invertase isoenzymes in sugarcane. Indian Sugar 56: 81–89.
Bhatia, S., Jyoti, S.K. Uppal, K.S. Thind, and S.K. Batta. 2009. Post harvest quality deterioration in sugarcane under different environmental conditions. Sugar Tech 11: 154–160.
Carson, D.L., and F.C. Botha. 2002. Genes expressed in sugarcane maturing internodal tissue. Plant Cell Reports 20: 1075–1081.
Chen, I.C.P. 1985. Cane Sugar Hand Book, 13–16. New York: Wiley Interscience Publication.
Gayler, K.R., and K.T. Glasziou. 1972. Physiological functions of acid and neutral invertases in growth and sugar storage in sugarcane. Physiologia Plantarum 27: 25–31.
Ghazi, I., L.F. Arrojo, H.G. Arellano, M. Ferrer, A. Ballesteros, and F.J. Plou. 2007. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. Journal of Biotechnology 128: 204–211.
Hocine, L.L., Z. Wang, B. Jiang, and S.J. Xu. 2000. Purification and partial characterization of a fructosyltransferase and invertase from Aspergillus niger AS0023. Journal of Biotechnology 81: 73–84.
Hussain, A., M.H. Rashid, R. Perveen, and M. Ashraf. 2009. Purification, kinetic and thermodynamic characterization of soluble acid invertase from sugarcane (Saccharum officinarium L.). Plant Physiology and Biochemistry 47: 188–194.
Isla, M.I., M.A. Vattuone, M.I. Gutierrez, and A.R. Sampietro. 1988. Acid invertase from Trapaeolum leaves. Phytochemistry 27: 1993–1998.
Karuppiah, N., B. Vadlamudi, and P.B. Kaufman. 1989. Purification and characterization of soluble (cytosolic) and bound (cell wall) isoforms of invertases in barley (Hordeum vulgare) elongating stem tissue. Plant Physiology 91: 993–998.
Kaur, S., S.K. Batta, J.S. Sital, K.P. Sharma, and A.P.S. Mann. 2002. Partial purification and properties of soluble invertase isoforms from sugarcane storage tissue. Indian Sugar 51: 851–857.
Lakshmanan, P., R.J. Geijskes, K.S. Aitken, C.P.L. Grof, G.D. Bonnett, and G.R. Smith. 2005. Sugarcane biotechnology: the challenges and opportunities. In Vitro Cellular and Developmental Biology Plant 41: 345–363.
Lineweaver, H., and D. Burk. 1934. The determination of enzyme dissociation constants. Journal of the American Chemical Society 56: 658–666.
Lingle, S.E. 1997. Seasonal internode development and sugar metabolism in sugarcane. Crop Science 37: 1222–1227.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265–275.
Mao, L., and W. Liu. 2000. Study on post harvest physiological changes and storage techniques of sugarcane. Scientia Agricultura Sinica 33: 41–45.
Mao, L., F. Que, and G. Wang. 2006. Sugar metabolism and involvement of enzymes in sugarcane (Saccharum officinarum L.) stem during storage. Food Chemistry 98: 338–342.
Nelson, N. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry 153: 375–380.
Prado, F.E., M.A. Vattuone, O.L. Fleishmacher, and A.R. Sampietro. 1985. Purification and characterization of Ricinus communis invertase. Journal of Biological Chemistry 260: 4952–4957.
Rosario, E.J.D., and V. Santisopasri. 1977. Characterization and inhibition of invertases in sugarcane juice. Phytochemistry 16: 443–445.
Sachdeva, M., A.P.S. Mann, and S.K. Batta. 2003. Multiple forms of soluble invertases in sugarcane juice: kinetic and thermodynamic analysis. Sugar Tech 5: 31–35.
Sachdeva, M., S. Bhatia, and S.K. Batta. 2011. Sucrose accumulation in sugarcane: a potential target for crop improvement. Acta Physiologiae Plantarum 33: 1571–1583.
Saxena, P., R.P. Srivastava, and M.L. Sharma. 2010. Impact of cut to crush delay and bio-chemical changes in sugarcane. Australian Journal of Crop Science 4: 692–699.
Solomon, S. 1994. Post harvest deterioration of sugarcane: physical and chemical methods to minimize inversion for higher sugar recovery. Indian Journal of Sugarcane Technology 9: 27–38.
Solomon, S., K.K. Srivastava, S. Bhatnagar, and V.K. Madan. 1990. Post harvest changes in invertase activity and juice quality in Sugarcane. Indian Sugar 39: 895–899.
Solomon, S., P. Singh, A.K. Shrivastava, P. Singh, A. Chandra, R. Jain, and C.P. Prajapati. 2011. Physico-chemical method of preserving sucrose in harvested sugarcane at high ambient temperature in a sub-tropical climate. Sugar Tech 13: 60–67.
Solomon, S. 2009. Post-harvest deterioration of sugarcane. Sugar Tech 11: 109–123.
Sturm, A. 1999. Invertases: Primary structures, functions and roles in plant development and sucrose partitioning. Plant Physiology 121: 1–8.
Vorster, D.J., and F.C. Botha. 1998. Partial purification and characterization of sugarcane neutral invertase. Phytochemistry 49: 651–655.
Zhu, Y.J., E. Komor, and P.H. Moore. 1997. Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiology 115: 609–616.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, S., Jyoti, Uppal, S.K. et al. Partial Purification and Characterization of Acid Invertase from the Fresh and Stale Sugarcane Juice. Sugar Tech 14, 148–155 (2012). https://doi.org/10.1007/s12355-012-0137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-012-0137-1