Abstract
Introduction
The efficacy of sugammadex on postoperative pulmonary complications (PPCs) in susceptible patients, compared with neostigmine, remains indeterminate. The Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) Group Investigators proposed a risk index for the early identification of susceptible patients, with excellent externally validated discrimination ability. Meta-analytical techniques were applied to evaluate the efficacy of sugammadex on PPCs in patients with ARISCAT-defined risk factors.
Methods
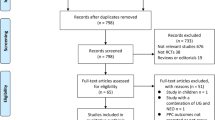
The study is registered on PROSPERO, number CRD42021261156. We searched PubMed, Scopus, Embase, Cochrane library, GreyNet, and OpenGrey for eligible randomized controlled trials (RCTs) without restricting the language or year of publication.
Results
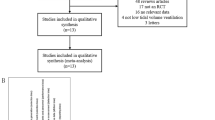
Twelve RCTs consisting of 1182 patients susceptible to PPCs were included. A robust reduction was observed on the incidence of PPCs in susceptible patients who received sugammadex [RR 0.66; 95% CI (0.54, 0.80), p < 0.01], with a low level of between-study heterogeneity (I2 = 45.98%; H2 = 1.85). Similar protective effects were also proved in avoiding residual neuromuscular block (NMB) [RR 0.25; 95% CI (0.11, 0.56); p < 0.01], atelectasis [RR 0.74; 95% CI (0.59, 0.95); p = 0.02], pneumonia [RR 0.49; 95% CI (0.28, 0.88); p = 0.02], and respiratory failure [RR 0.61; 95% CI (0.39, 0.96); p = 0.03]. No difference was observed regarding adverse events [RR 0.85; 95% CI (0.72, 1.01); p = 0.06].
Conclusion
Low to moderate quality of evidence demonstrated the edge of sugammadex over neostigmine for NMB reversal in reducing the likelihood of PPCs and residual NMB in patients with ARISCAT-defined risk factors. Clinicians may reassess the type of reversal agent when treating patients susceptible to PPCs.





Similar content being viewed by others
References
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118:317–34.
McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005;171:514–7.
Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41 (discussion 41–3).
Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–50.
Rahe-Meyer N, Berger C, Wittmann M, et al. Recovery from prolonged deep rocuronium-induced neuromuscular blockade: a randomized comparison of sugammadex reversal with spontaneous recovery. Anaesthesist. 2015;64:506–12.
Jones RK, Caldwell JE, Brull SJ, et al. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24.
Lawrence VA, Hilsenbeck SG, Mulrow CD, et al. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10:671–8.
Canet J, Sabaté S, Mazo V, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32:458–70.
Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219–31.
Jin Y, Xie G, Wang H, et al. Incidence and risk factors of postoperative pulmonary complications in noncardiac Chinese patients: a multicenter observational study in university hospitals. Biomed Res Int. 2015;2015: 265165.
Kokotovic D, Degett TH, Ekeloef S, et al. The ARISCAT score is a promising model to predict postoperative pulmonary complications after major emergency abdominal surgery: an external validation in a Danish cohort. Eur J Trauma Emerg Surg. 2022;48:3863–7.
Murphy GS, Szokol JW, Marymont JH, et al. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology. 2008;109:389–98.
Hristovska AM, Duch P, Allingstrup M, et al. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018;73:631–41.
Kirmeier, Leva B, Harlet P, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7:129–40.
Krause M, McWilliams SK, Bullard KJ, et al. Neostigmine versus sugammadex for reversal of neuromuscular blockade and effects on reintubation for respiratory failure or newly initiated noninvasive ventilation: an interrupted time series design. Anesth Analg. 2020;131:141–51.
Kheterpal S, Vaughn MT, Dubovoy TZ, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132:1371–81.
Colquhoun DA, Vaughn MT, Bash LD, et al. Association between choice of reversal agent for neuromuscular block and postoperative pulmonary complications in patients at increased risk undergoing non-emergency surgery: STIL-STRONGER, a multicentre matched cohort study. Br J Anaesth. 2023;130:e148–59.
Wang JF, Zhao ZZ, Jiang ZY, et al. Influence of sugammadex versus neostigmine for neuromuscular block reversal on the incidence of postoperative pulmonary complications: a meta-analysis of randomized controlled trials. Perioper Med (Lond). 2021;10(1):32.
Yağan Ö, Taş N, Mutlu T, et al. Comparison of the effects of sugammadex and neostigmine on postoperative nausea and vomiting. Braz J Anesthesiol. 2017;67:147–52.
Hakimoğlu S, Tuzcu K, Davarcı I, et al. Comparison of sugammadex and neostigmine-atropine on intraocular pressure and postoperative effects. Kaohsiung J Med Sci. 2016;32:80–5.
Geldner G, Niskanen M, Laurila P, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67:991–8.
Ba YF, Liu YN, He SH, et al. Analysis of sugammadex for antagonistic neuromuscular block in patients with radical resection of lung cancer under thoracoscope. Zhonghua Yi Xue Za Zhi. 2020;100:213–9.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6: e1000100.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105.
Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (Updated March 2011). Cochrane handbook for systematic reviews of interventions.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Jakobsen JC, Gluud C, Winkel P, et al. The thresholds for statistical and clinical significance - a five-step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol. 2014;14:34.
Takwoingi Y, Hopewell S, Tovey D, et al. A multicomponent decision tool for prioritising the updating of systematic reviews. BMJ. 2013;347: f7191.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Yu Y, Wang H, Bao Q, et al. Sugammadex versus neostigmine for neuromuscular block reversal and postoperative pulmonary complications in patients undergoing resection of lung cancer. J Cardiothorac Vasc Anesth. 2022;36:3626–33.
Williams WH 3rd, Cata JP, Lasala JD, et al. Effect of reversal of deep neuromuscular block with sugammadex or moderate block by neostigmine on shoulder pain in elderly patients undergoing robotic prostatectomy. Br J Anaesth. 2020;124:164–72.
Togioka BM, Yanez D, Aziz MF, et al. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020;124:553–61.
Olesnicky B, Doane M, Farrell C, et al. Prevention of postoperative events following reversal with sugammadex or neostigmine (the P-PERSoN Trial): pilot data following early termination of a prospective, blinded, randomised trial. Anesthesiol Res Pract. 2022;2022:4659795.
Lee YJ, Oh AY, Koo BW, et al. Postoperative residual neuromuscular blockade after reversal based on a qualitative peripheral nerve stimulator response: a randomised controlled trial. Eur J Anaesthesiol. 2020;37:196–202.
Ledowski T, Szabó-Maák Z, Loh PS, et al. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: a prospective, randomised, double-blind trial in high-risk older patients. Br J Anaesth. 2021;127:316–23.
Evron S, Abelansky Y, Ezri T, et al. Respiratory events with sugammadex vs. neostigmine following laparoscopic sleeve gastrectomy: a prospective pilot study assessing neuromuscular reversal strategies. Rom J Anaesth Intensive Care. 2017;24:111–4.
Çitil AB, Tuncel ZA, Yapici N, et al. Reversal of rocuronium induced neuromuscular blockade in lung resection surgery: A comparison of sugammadex and neostigmine. Gogus-Kalp-Damar Anestezi ve Yogun Bakim Dernegi Dergisi. 2019;25:23–30.
Carron M, Veronese S, Foletto M, et al. Sugammadex allows fast-track bariatric surgery. Obes Surg. 2013;23:1558–63.
Brueckmann B, Sasaki N, Grobara P, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115:743–51.
Alday E, Muñoz M, Planas A, et al. Effects of neuromuscular block reversal with sugammadex versus neostigmine on postoperative respiratory outcomes after major abdominal surgery: a randomized-controlled trial. Can J Anaesth. 2019;66:1328–37.
Han J, Oh AY, Jeon YT, et al. Quality of recovery after laparoscopic cholecystectomy following neuromuscular blockade reversal with neostigmine or sugammadex: a prospective, randomized, controlled trial. J Clin Med. 2021;10:1–10.
Hurford WE, Welge JA, Eckman MH. Sugammadex versus neostigmine for routine reversal of rocuronium block in adult patients: a cost analysis. J Clin Anesth. 2020;67: 110027.
Carron M, Baratto F, Zarantonello F, et al. Sugammadex for reversal of neuromuscular blockade: a retrospective analysis of clinical outcomes and cost-effectiveness in a single center. Clinicoecon Outcomes Res. 2016;8:43–52.
Brett K, Farrah K. CADTH rapid response reports. Sugammadex for the reversal of neuromuscular blockade in surgical patients: a review of clinical effectiveness and cost-effectiveness. Ottawa: Canadian Agency for Drugs and Technologies in Health. Copyright © 2019 Canadian agency for drugs and technologies in health.; 2019.
Berg H. Is residual neuromuscular block following pancuronium a risk factor for postoperative pulmonary complications? Acta Anaesthesiol Scand Suppl. 1997;110:156–8.
Nieuwenhuijs D, Bruce J, Drummond GB, et al. Ventilatory responses after major surgery and high dependency care. Br J Anaesth. 2012;108:864–71.
Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120–8.
Cedborg AI, Sundman E, Bodén K, et al. Pharyngeal function and breathing pattern during partial neuromuscular block in the elderly: effects on airway protection. Anesthesiology. 2014;120:312–25.
D’Honneur G, Lofaso F, Drummond GB, et al. Susceptibility to upper airway obstruction during partial neuromuscular block. Anesthesiology. 1998;88:371–8.
Dobson G, Chow L, Filteau L, et al. Guidelines to the practice of anesthesia—revised edition 2020. Can J Anaesth. 2020;67:64–99.
Naguib M, Brull SJ, Kopman AF, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127:71–80.
Checketts MR, Alladi R, Ferguson K, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2016;71:85–93.
Thilen SR, Weigel WA, Todd MM, et al. 2023 American Society of Anesthesiologists Practice Guidelines for monitoring and antagonism of neuromuscular blockade: a report by the american society of anesthesiologists task force on neuromuscular blockade. Anesthesiology. 2023;138:13–41.
Fuchs-Buder T, Romero CS, Lewald H, et al. Peri-operative management of neuromuscular blockade: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol EJA. 2023;40:82–94.
Eleveld DJ, Kuizenga K, Proost JH, et al. A temporary decrease in twitch response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg. 2007;104:582–4.
Le Corre F, Nejmeddine S, Fatahine C, et al. Recurarization after sugammadex reversal in an obese patient. Can J Anaesth. 2011;58:944–7.
Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345: e6329.
Payne JP, Hughes R, Al AS. Neuromuscular blockade by neostigmine in anaesthetized man. Br J Anaesth. 1980;52:69–76.
Yost CS, Maestrone E. Clinical concentrations of edrophonium enhance desensitization of the nicotinic acetylcholine receptor. Anesth Analg. 1994;78:520–6.
Legendre P, Ali DW, Drapeau P. Recovery from open channel block by acetylcholine during neuromuscular transmission in zebrafish. J Neurosci. 2000;20:140–8.
Herbstreit F, Zigrahn D, Ochterbeck C, et al. Neostigmine/glycopyrrolate administered after recovery from neuromuscular block increases upper airway collapsibility by decreasing genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2010;113:1280–8.
Bronsert MR, Henderson WG, Monk TG, et al. Intermediate-acting nondepolarizing neuromuscular blocking agents and risk of postoperative 30-day morbidity and mortality, and long-term survival. Anesth Analg. 2017;124:1476–83.
Bulka CM, Terekhov MA, Martin BJ, et al. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology. 2016;125:647–55.
Keating GM. Sugammadex: a review of neuromuscular blockade reversal. Drugs. 2016;76:1041–52.
Acknowledgements
Funding
This study was funded by the National Key Research and Development Program of China (Grant No. 2018YFC2001903) and the National Natural Science Foundation of China (Grant No. 81873952) and the National Natural Science Foundation of China (Grant No. 81901948). The Rapid Service Fee was funded by the authors.
Author Contributions
All named authors were involved in study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by Yun-Xiao Bai, Jing-Jing Han, Ke-Xuan Liu and Qing-Ping Wu, and all authors provided comments on subsequent drafts. All authors read and approved the final manuscript.
Disclosures
Yun-Xiao Bai, Jin-Jing Han, Jie Liu, Xia Li, Zhen-Zhen Xu, Yong Lv, Ke-Xuan Liu, Qing-Ping Wu have nothing to disclose.
Compliance with Ethics Guidelines
Since this article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, no ethics committee approval is required for this study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, YX., Han, JJ., Liu, J. et al. Sugammadex Reduced the Incidence of Postoperative Pulmonary Complications in Susceptible Patients Identified by ARISCAT Risk Index: Systematic Review and Meta-analysis. Adv Ther 40, 3784–3803 (2023). https://doi.org/10.1007/s12325-023-02535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02535-9