Abstract
Introduction
The blood pressure (BP) control mechanism for mineralocorticoid receptor blockers is unclear, and analysis of their use as a single agent in the clinical setting is required to resolve this uncertainty. There is a paucity of data on esaxerenone monotherapy assessing its long-term antihypertensive effect and urinary biomarkers.
Methods
This post hoc exploratory substudy of a long-term phase 3 study evaluated the effect of esaxerenone monotherapy (2.5 or 5 mg/day) in treatment-naïve patients who continued the therapy during the 52-week study period (n = 25). In addition to blood biomarkers, urinary biomarkers were also assessed in 24-h urine collection samples.
Results
Esaxerenone monotherapy was associated with consistent reductions in systolic/diastolic BP in the substudy population (− 23.5/− 13.1 mmHg at week 52, p < 0.001 vs baseline). Plasma aldosterone concentrations and plasma renin activity significantly increased during esaxerenone monotherapy at all time points. On the basis of the observations that both urine volume and urinary sodium excretion also decreased up to the end of the study, and were significantly lower at 12 weeks, patients were further categorized into higher/lower urinary sodium excretion subgroups according to whether their baseline values were above or below the median. In the group with higher baseline urinary sodium excretion, esaxerenone exhibited a significantly greater decrease in systolic/diastolic BP compared to the lower baseline group.
Conclusion
Esaxerenone exhibited sustained and stable antihypertensive activity even when administered as a single agent for 52 weeks in patients with essential hypertension. The additional urinary biomarker analysis suggests that the BP-lowering effects of esaxerenone may be partly exerted via mechanisms related to salt and water retention, and that the effect is particularly pronounced in patients with hypertension and higher baseline urinary sodium excretion, which may reflect a state of excessive salt intake.
Trial registration
NCT02722265.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
How mineralocorticoid receptor (MR) blockers control blood pressure (BP) is not well understood. |
Analyzing long-term single use of an MR blocker and relevant biomarkers (blood and urinary) could provide insight into these mechanisms. |
This post hoc exploratory substudy investigated the antihypertensive effect of esaxerenone and urinary biomarkers in treatment-naïve patients who continued esaxerenone monotherapy (2.5 or 5 mg/day) for 52 weeks. |
What was learned from the study? |
Esaxerenone is expected to have a sustained and stable antihypertensive effect even in a long-term monotherapy, with increases in plasma aldosterone concentrations and plasma renin activity. |
The additional urinary biomarker analysis provided a new perspective on the clinical effects of esaxerenone that its BP-lowering effect may be enhanced in patients with higher baseline urinary sodium excretion via mechanisms related to salt and water retention. |
The favorable antihypertensive effect in patients with higher baseline sodium excretion suggests that esaxerenone may be a useful new treatment option for patients with hypertension and excessive salt intake. |
Introduction
Mineralocorticoid receptors (MRs) are expressed in distal convoluted ducts and collecting duct epithelial cells. They promote sodium reabsorption and potassium excretion through aldosterone binding, and regulate blood electrolyte mass and circulating blood volume within the normal range. Excessive aldosterone leads to excessive MR signal activation, causing an increase in circulating blood volume and hypertension [1, 2]. Excessive salt ingestion also stimulates MRs in the kidney, causing salt-induced hypertension [3]. In an animal model, one of the mechanisms for salt-induced hypertension has been clarified; that is, excessive salt intake enhanced MR signaling independent of aldosterone, via Rac1, which promotes hypertension and renal damage [4]. Other studies have also shown that MR blockade produces antihypertensive and organ-protective effects in salt-loaded animal models [5,6,7,8]. As was observed in these studies, in clinical practice, stronger antihypertensive effects of MR blockers were observed in patients with high salt intake or low renin [9, 10], and urinary sodium excretion has been reported to correlate with the antihypertensive effect of spironolactone [11]. These findings indicate that the inhibition of MR activity by MR blocker is likely to potentiate its antihypertensive effect in patients with hypertension and excessive salt intake. However, there is a paucity of literature evaluating the relationship between the antihypertensive effect of MR blockers and sodium excretion so far.
Esaxerenone (MINNEBRO®, Daiichi Sankyo Co., Ltd.), a novel selective MR blocker with a non-steroidal structure, was approved in Japan in January 2019 for the treatment of hypertension [12, 13]. Esaxerenone has shown good antihypertensive activity in patients with uncomplicated grade I–III hypertension, hypertension with moderate renal impairment, hypertension with type 2 diabetes mellitus with albuminuria, and hypertension associated with primary aldosteronism [14,15,16,17,18,19].
Among a series of clinical studies on esaxerenone, a long-term phase 3 clinical study showed that esaxerenone was effective and well tolerated as an add-on therapy to a calcium channel blocker (CCB) or renin–angiotensin system (RAS) inhibitor in Japanese patients with essential hypertension [15]. Unusually in the context of assessing the long-term antihypertensive effects of a single agent, the study analyzed 24-h urinary samples. In this substudy, we investigated whether there is an association between the antihypertensive effect of esaxerenone and urinary biomarkers in treatment-naïve patients who received esaxerenone monotherapy (2.5 or 5 mg/day) for 52 weeks and had biomarker data available.
Methods
Study Design
This analysis was a post hoc exploratory substudy of a multicenter, open-label, long-term phase 3 study of esaxerenone added to treatment with a CCB or RAS inhibitor in patients with essential hypertension (NCT02722265) [15]. The main and substudy protocols were approved by the relevant institutional review boards (Supplementary Material Table S1), and all study procedures complied with the ethical principles of the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent prior to enrollment.
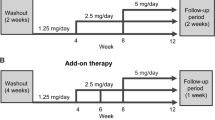
The main study consisted of a 4-week baseline observation period, followed by two treatment periods (baseline to week 12, and week 12 to week 28 or 52) (Supplementary Material Fig. S1) [15]. The date of consent for the first patient was March 4, 2016, and the final observation date was July 8, 2017.
Study Population
Full details of the main study inclusion criteria have been reported previously [15]. Briefly, patients had essential hypertension, an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2, and had not received any prior antihypertensive drug therapy or were receiving a CCB or RAS inhibitor monotherapy. Patients with primary aldosteronism (secondary hypertension) were excluded from this study. Patients not taking antihypertensive medications were allocated to the esaxerenone monotherapy group, whereas those currently receiving a CCB or RAS inhibitor had esaxerenone added to existing therapy (combination therapy group).
This post hoc exploratory substudy included patients from the esaxerenone monotherapy group with biomarker data available who remained on monotherapy for the full 52 weeks of treatment (i.e., did not require the addition of other antihypertensive agents after week 12 to achieve blood pressure [BP] control). When interpreting the results obtained with esaxerenone monotherapy, we observed a significant change in sodium excretion. Therefore, an additional subanalysis by sodium excretion was performed; i.e., patients were further categorized into higher or lower baseline urinary sodium excretion subgroups according to whether their baseline values were above or below the median.
Study Treatments
After initiation of esaxerenone at 2.5 mg/day, the dosage could be increased to 5 mg/day at weeks 4, 6, or 8, as required, to achieve the target systolic BP/diastolic BP of < 140/< 90 mmHg, or < 130/< 80 mmHg in patients with diabetes [15]. In the main study, from week 12 onwards, increasing the dosage of existing antihypertensives or the addition of other antihypertensive agents was permitted if needed to achieve BP control. However, this substudy excluded patients receiving any additional hypertensives to focus on those receiving esaxerenone alone.
During the period from the start date of the observation period to the end of the post-observation period, conventional dietary habits (including foods rich in potassium) were to remain constant, where possible. There were no strict restrictions on salt or water intake. The following concomitant drugs were prohibited from 4 weeks prior to the start date of the treatment period to the end date of the treatment period or the time of discontinuation: antihypertensive drugs, diuretics, non-steroidal anti-inflammatory drugs, insulin preparations, potassium preparations, ion-exchange resins, licorice, and other products containing glycyrrhizin.
Biomarker Measurements
Blood and urine samples were collected at baseline; weeks 12, 28, and 52; and at the time of therapy discontinuation. Blood was collected in the supine position after a supine rest period of no less than 30 min. Urine samples were collected by 24-h sampling using Urinmate® P (Sumitomo Bakelite Co. Ltd., Tokyo, Japan), a 24-h urine collection instrument that collects 24-h urine at a 1:50 volume ratio. Twenty-four-hour urine collection was started from the time of awakening on the day before each scheduled visit day to the same time on the visit day. Of note, to explore the mechanism of action of esaxerenone based on biomarker variability, 24-h urine sampling was prespecified only in the monotherapy group.
Blood and urine samples were used to determine the following parameters at each time point: renin–angiotensin–aldosterone system-related biomarkers, including plasma aldosterone concentration (PAC) and plasma renin activity (PRA); daily (24-h) urinary sodium excretion, and urinary creatinine; human atrial natriuretic peptide (hANP) and N-terminal pro-brain natriuretic peptide (NT-proBNP); eGFR; and serum potassium. Daily (24-h) urinary sodium excretion was calculated using the formula urine volume × urine concentration. Sample collection and laboratory tests were performed by LSI Medience (Tokyo, Japan).
Statistical Analysis
Missing data were not imputed. Changes in BP and biomarkers from baseline to each time point were calculated; a paired t test was used to compare pre- and post-treatment values. Log-transformed measurements were used to calculate point estimates and 95% confidence intervals for the geometric mean percentage change from baseline at each time point. The t test was also used to compare differences in change from baseline at each time point in patient subgroups based on higher or lower baseline urinary sodium excretion. Statistical tests were not adjusted for multiplicity because of the explanatory nature of this substudy. All analyses were conducted using SAS System Release 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of the 245 patients who received esaxerenone monotherapy in the main long-term phase 3 study, 102 continued the 52-week therapy, of whom 57 had biomarker data available. Of these, 25 patients who remained on esaxerenone monotherapy until week 52 were included in the current analysis (Supplementary Material Table S2); none were taking concomitant antihypertensive medication.
Baseline characteristics did not differ markedly between the present substudy group and the main long-term phase 3 study. Urinary biomarkers from 24-h urine collection were not measured in all patients of the long-term study (Table 1 and Supplementary Material Table S3).
Effects of Esaxerenone on BP and Biomarkers
Esaxerenone monotherapy was associated with consistent reductions in systolic BP/diastolic BP in the substudy population (− 20.8/− 10.3, − 27.6/− 15.2, and − 23.5/− 13.1 mmHg at 12, 28, and 52 weeks, respectively; p < 0.001 vs baseline) (Fig. 1 and Supplementary Material Table S4). The antihypertensive effect of monotherapy was similar to that observed in the main study (Fig. 1).
PAC and PRA significantly increased during therapy with esaxerenone at all time points compared with baseline (all p < 0.001 vs baseline, except for PRA at week 28, p < 0.05), and this increase remained constant after 12 weeks (Supplementary Material Table S4). Although the patients in this study did not have any comorbidities associated with renal- or heart failure-related diseases and the values were within the normal range, both hANP and NT-proBNP decreased, and the decrease at 52 weeks was significant (change from baseline at week 52: hANP, − 5.5 pg/mL, p < 0.05 vs baseline; NT-proBNP, − 46.8 pg/mL, p < 0.05 vs baseline) (Supplementary Material Table S4). The eGFR also decreased overall (Supplementary Material Table S4).
Effects of Esaxerenone on Urinary Sodium and Potassium Excretion
In the first 12 weeks, urinary sodium excretion, potassium excretion, and urine volume decreased significantly (all p < 0.05 vs baseline), and the decrease was sustained until 52 weeks (Supplementary Material Table S4). Spaghetti plots of the respective changes in these parameters from baseline to each time point are shown in Fig. 2. Urine volume decreased at 12, 28, and 52 weeks compared with baseline as follows: − 444.8 mL (geometric mean change, − 26.0%), − 83.0 mL (− 6.7%), and − 319.0 mL (− 18.6%), respectively (Fig. 2a and Supplementary Material Table S4). There was no statistically significant change in urinary sodium concentration, which was 8.9 (geometric mean change, 2.1%), − 7.7 (− 9.0%), and 3.1 (− 0.4%) mEq/L at 12, 28, and 52 weeks, respectively (Fig. 2b and Supplementary Material Table S4). Urinary sodium excretion decreased through 52 weeks, with statistically significant changes at 12 weeks (− 53.1 mEq/day; geometric mean change, − 24.5%, p = 0.0019 vs baseline) and 52 weeks (− 44.0 mEq/day; geometric mean change, − 18.9%, p = 0.0307 vs baseline) (Fig. 2c and Supplementary Material Table S4). Thus, the decrease in urinary sodium excretion could be attributed to the decrease in urine volume.
In line with the results for urinary sodium, urinary potassium concentrations did not change notably at 12, 28, or 52 weeks compared with baseline (Fig. 2d and Supplementary Material Table S4). The mean change in urinary potassium excretion tended to decrease from baseline to − 12.2 (geometric mean change, − 21.5%), − 4.4 (− 14.5%), and − 4.3 (− 8.5%) mEq/day at 12, 28, and 52 weeks, respectively, but the change was statistically significant only at 12 weeks (p = 0.0092 vs baseline) (Fig. 2e and Supplementary Material Table S4). This decreased urinary potassium excretion was also attributed to decreased urine volume. Although the urinary sodium/potassium (Na/K) ratio remained constant during 12 (Δ − 0.3 [− 3.8%]), 28 (Δ − 0.1 [− 0.7%]), and 52 (Δ − 0.5 [− 11.4%]) weeks of esaxerenone administration, the ratio tended to decrease compared with baseline (Fig. 2f and Supplementary Material Table S4).
Effects of Esaxerenone in the Urinary Sodium Excretion Subgroup
To clarify the relationship between the antihypertensive effect of esaxerenone and urinary sodium excretion, patients were divided into two groups according to baseline urinary sodium excretion. Urinary sodium excretion was higher than the median value of 193.8 mEq/day in 13 patients and lower than the median value in 12 patients (Supplementary Material Tables S3 and S4).
Baseline characteristics did not differ markedly between patient subgroups based on baseline urinary sodium excretion. Numerical differences were higher male ratio, urine volume, urinary potassium excretion, and Na/K ratio in the higher baseline urinary sodium excretion subgroup versus the lower subgroup (Supplementary Material Table S3).
At 12 weeks, BP decreased significantly in both the lower and higher baseline urinary sodium excretion subgroups (− 14.9/− 7.3 and − 26.6/− 13.2 mmHg, respectively; both p < 0.001 vs baseline); however, there was a significantly greater decrease in the higher baseline urinary sodium excretion subgroup vs the lower baseline urinary sodium excretion subgroup (p < 0.05 for the difference in systolic BP and diastolic BP at 12 weeks between the subgroups) (Fig. 3 and Supplementary Material Table S4).
In the group with higher baseline urinary sodium excretion, urinary sodium excretion, and urine volume decreased with significant differences, and Na/K ratio also decreased. In addition, PRA was consistently slightly elevated throughout the study. There were no other differences (Supplementary Material Table S4).
Discussion
In this post hoc exploratory substudy conducted in patients with essential hypertension who participated in a phase 3 clinical study of esaxerenone [15], we investigated the antihypertensive effect of long-term monotherapy with esaxerenone along with various biomarkers, including PAC, PRA, and urinary sodium excretion. We then examined the relationship between its antihypertensive effect and baseline urinary sodium excretion. The results have elicited important new information related to the use of esaxerenone monotherapy in patients who did not take other antihypertensive drugs that affect urinary sodium excretion throughout the study.
The reduction in systolic BP/diastolic BP at the end of 52 weeks of therapy in the subset of patients who continued esaxerenone monotherapy was − 23.5/− 13.1 mmHg, which was consistent with the decrease in BP seen in the overall patient group of the main phase 3 long-term study (− 23.1/− 12.5 mmHg) [15]. There was also no difference in BP reduction compared to the three treatment groups in the main phase 3 study; esaxerenone monotherapy (− 23.7/− 12.3 mmHg) and combination of RAS inhibitor (− 23.0/− 12.6 mmHg) or CCB (− 20.5/− 13.1 mmHg) [15]. In the current analysis, the BP-lowering effects of esaxerenone at week 12 were greater in the subgroup of patients with higher urinary sodium excretion at baseline, presumably because of their initial high salt loading status. These results are similar to those reported for existing MR blockers. In a previous clinical study, eplerenone exhibited strong antihypertensive activity in the highest tertiles of the salt intake level [9], and data from another substudy showed that the BP-lowering effects of spironolactone were greater in patients with low baseline PRA [20], one of the indirect markers of excessive salt intake [21,22,23]. Thus, the stronger antihypertensive activity of MR blockers in patients with higher salt intake suggests that their BP-lowering effect is exerted via mechanisms related to salt and water retention.
Aldosterone is a hormone that retains sodium, and when aldosterone is deficient, the body loses sodium [24]. Therefore, sodium excretion should have been increased by the blocking of MRs with esaxerenone administration in this study. However, contrary to our expectations, sodium excretion decreased after 12 weeks and up to the end of the study. We speculate that this may be due to the normalization of sodium retention by elimination of excess sodium accumulated in the body during the short period immediately after esaxerenone administration. The time required for this normalization of sodium retention is assumed to be 2 weeks. The rationale for this is that elevated serum potassium levels only occurred in the first 2 weeks after treatment initiation in the long-term main study [15, 25], and BP also decreases after 2 weeks of esaxerenone administration [15]. Moreover, in rats, the urinary Na/K ratio shows an increase at just 3 h after esaxerenone administration [26]. We deduced that up to 2 weeks after esaxerenone administration, sodium excretion is transiently increased and excess sodium is sufficiently eliminated from the body. As a result, at 12 weeks, the amount of excreted sodium has already decreased compared with that before esaxerenone administration, and there is no longer a need to eliminate excess sodium from the body. Therefore, it may have appeared that sodium excretion was decreased after 12 weeks post-dose compared with pre-dose. The apparent decrease in sodium excretion in the higher sodium excretion group compared with the lower sodium excretion group at baseline also supports this hypothesis. Furthermore, albeit within normal range, a significant reduction in hANP, which is released from the atrial wall with its stretch stimulus and reduces fluid volume by inhibiting the excretion of renin and aldosterone [27], also indicates a decrease in circulating blood volume along with the reduction in BP. To summarize, at least part of the antihypertensive action of MR blockers may be attributed to a decrease in circulating blood volume due to the cancellation of excess sodium retention by the elimination of sodium from the body. To further confirm the hypothesis, it would be necessary to collect additional data on urinary sodium excretion immediately after esaxerenone administration, especially within 2 weeks, which can be considered a study limitation.
Esaxerenone decreased the Na/K ratio, consistent with a previous study reporting that BP decreases as the urinary Na/K ratio decreases [28]. Other studies reported that the Na/K ratio is more strongly associated with the risk of cardiovascular disease than sodium and potassium excretion [29, 30]. Currently, there is no consensus on the threshold of urinary Na/K levels at which the risk of cardiovascular events increases, but esaxerenone may reduce the risk of cardiovascular disease in the long term by decreasing the urinary Na/K ratio. The potential for esaxerenone to reduce the risk of cardiovascular diseases can also be inferred from changes in NT-proBNP, a marker of heart failure. In this study, NT-proBNP was significantly reduced after esaxerenone administration, although still within the normal range. This finding is consistent with data from previous studies in patients with chronic heart failure and diabetes and/or chronic kidney disease who were treated with the MR blockers finerenone or eplerenone [31].
In Japan, angiotensin receptor blockers/CCBs are prescribed as the main antihypertensive drugs, with these two drugs accounting for approximately 90% of prescriptions [32]. Since it is difficult to identify patients with hypertension and excessive salt intake in daily practice, it can be assumed that many of these patients are treated with RAS inhibitors. However, the antihypertensive effect of RAS inhibitors is attenuated by high salt intake [33,34,35], so the efficacy of RAS inhibitors may be inadequate in patients with hypertension and excessive salt intake. The current guideline recommends diuretics for treating patients with excessive salt intake [36], but their metabolic side effects, including hyperuricemia and impaired glucose tolerance, are known to increase especially at higher doses. Therefore, MR blockers, which normalize sodium retention, are expected to be another good antihypertensive treatment option for patients with excessive salt intake.
Limitations
First, this was a post hoc exploratory substudy, and data were analyzed using point-by-point t tests without considering multiplicity of tests. In addition, the study was conducted in a small number of patients from a larger clinical study, and it had low statistical power for comparisons between even smaller patient subgroups based on baseline urinary sodium excretion. However, it provides important insights for planning the next validation study. Second, we could not exclude the effects of potential confounders on our findings, given the self-selected (non-randomized) nature of the substudy population. Third, the study did not measure the amount of urine and urinary/blood sodium level immediately after esaxerenone administration (e.g., on the first day/week after treatment administration). Fourth, dietary salt and water intakes were not strictly controlled or measured, although patients were instructed to carry on as usual in the study. Finally, the patients in this study were registered within a similar time period in the year (during the spring season). It is known that urine and other body fluid volumes are affected by seasonal changes [37].
Conclusion
In this post hoc exploratory substudy, the antihypertensive effect of esaxerenone was evaluated without concomitant use of other antihypertensive drugs for 52 weeks. Esaxerenone monotherapy demonstrated a sustained and stable antihypertensive effect in patients with essential hypertension. The additional urinary biomarker subanalysis provided a new perspective on the clinical effects of esaxerenone that its BP-lowering effect may be mediated by salt and water retention. The favorable antihypertensive effect in patients with higher baseline sodium excretion indicates that esaxerenone may be a new and first-line treatment option for patients with hypertension due to excessive salt intake. These findings suggest that esaxerenone may play a key role in the treatment of hypertension and may, therefore, represent an important therapeutic strategy in the near future.
References
Terker AS, Ellison DH. Renal mineralocorticoid receptor and electrolyte homeostasis. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1068–70. https://doi.org/10.1152/ajpregu.00135.2015.
Esteras R, Perez-Gomez MV, Rodriguez-Osorio L, Ortiz A, Fernandez-Fernandez B. Combination use of medicines from two classes of renin-angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf. 2015;6:166–76. https://doi.org/10.1177/2042098615589905.
Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med. 2012;44(1 Suppl):S119–26. https://doi.org/10.3109/07853890.2012.671538.
Shibata S, Mu S, Kawarazaki H, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43. https://doi.org/10.1172/JCI43124.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73. https://doi.org/10.1016/j.ejphar.2015.11.028.
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther. 2016;358:548–57. https://doi.org/10.1124/jpet.116.234765.
Li L, Guan Y, Kobori H, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res. 2019;42:769–78. https://doi.org/10.1038/s41440-018-0187-1.
Bhuiyan AS, Rafiq K, Kobara H, Masaki T, Nakano D, Nishiyama A. Effect of a novel nonsteroidal selective mineralocorticoid receptor antagonist, esaxerenone (CS-3150), on blood pressure and renal injury in high salt-treated type 2 diabetic mice. Hypertens Res. 2019;42:892–902. https://doi.org/10.1038/s41440-019-0211-0.
Nishimoto M, Ohtsu H, Marumo T, et al. Mineralocorticoid receptor blockade suppresses dietary salt-induced ACEI/ARB-resistant albuminuria in non-diabetic hypertension: a sub-analysis of evaluate study. Hypertens Res. 2019;42:514–21. https://doi.org/10.1038/s41440-018-0201-7.
Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75. https://doi.org/10.1161/CIRCULATIONAHA.107.690396.
Ghazi L, Dudenbostel T, Lin CP, Oparil S, Calhoun DA. Urinary sodium excretion predicts blood pressure response to spironolactone in patients with resistant hypertension independent of aldosterone status. J Hypertens. 2016;34:1005–10. https://doi.org/10.1097/HJH.0000000000000870.
Minnebro® (esaxerenone) tablets 1.25 mg, 2.5 mg, 5 mg [package insert]. Japan: Daiichi-Sankyo; 2021. Accessed 17 Jan 2021 https://pins.japic.or.jp/pdf/newPINS/00067892.pdf. Japanese.
Duggan S. Esaxerenone: first global approval. Drugs. 2019;79:477–81. https://doi.org/10.1007/s40265-019-01073-5.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75:51–8. https://doi.org/10.1161/HYPERTENSIONAHA.119.13569.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42:1932–41. https://doi.org/10.1038/s41440-019-0314-7.
Rakugi H, Ito S, Ito H, Okuda Y, Iijima S. The efficacy and safety of esaxerenone for patients with grade III hypertension. Prog Med. 2020;40:755–60.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44:489–97. https://doi.org/10.1038/s41440-020-00585-y.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81. https://doi.org/10.1038/s41440-019-0270-2.
Satoh F, Ito S, Itoh H, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44:464–72. https://doi.org/10.1038/s41440-020-00570-5.
Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68. https://doi.org/10.1016/S0140-6736(15)00257-3.
Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2020;12:4022. https://doi.org/10.1002/14651858.CD004022.pub3.
Laragh JH, Sealey JE. The plasma renin test reveals the contribution of body sodium-volume content (V) and renin-angiotensin (R) vasoconstriction to long-term blood pressure. Am J Hypertens. 2011;24:1164–80. https://doi.org/10.1038/ajh.2011.171.
He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension. 2001;38:321–5. https://doi.org/10.1161/01.hyp.38.3.321.
Ayuzawa N, Fujita T. The mineralocorticoid receptor in salt-sensitive hypertension and renal injury. J Am Soc Nephrol. 2021;32:279–89. https://doi.org/10.1681/ASN.2020071041.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85. https://doi.org/10.1038/s41440-020-00569-y.
Arai K, Homma T, Morikawa Y, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34. https://doi.org/10.1016/j.ejphar.2015.06.015.
Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci. 2016;130:57–77. https://doi.org/10.1042/CS20150469.
Kogure M, Nakaya N, Hirata T, et al. Sodium/potassium ratio change was associated with blood pressure change: possibility of population approach for sodium/potassium ratio reduction in health checkup. Hypertens Res. 2021;44:225–31. https://doi.org/10.1038/s41440-020-00536-7. (Erratum published in Hypertens Res. 2021;44:262. https://doi.org/10.1038/s41440-020-00547-4).
Okayama A, Okuda N, Miura K, et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open. 2016;6: e011632. https://doi.org/10.1136/bmjopen-2016-011632.
Cook NR, Obarzanek E, Cutler JA, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch of Intern Med. 2009;169:32–40. https://doi.org/10.1001/archinternmed.2008.523.
Sato N, Ajioka M, Yamada T, et al. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circ J. 2016;80:1113–22. https://doi.org/10.1253/circj.CJ-16-0122.
Ibaraki A, Goto W, Iura R, Tominaga M, Tsuchihashi T. Current prescription status of antihypertensive drugs with special reference to the use of diuretics in Japan. Hypertens Res. 2017;40:203–6. https://doi.org/10.1038/hr.2016.120.
Kai H. Antihypertensive drug therapy for salt-sensitive hypertension. Prog Med. 2012;32:1047–50.
Kimura G, Deguchi F, Kojima S, et al. Antihypertensive drugs and sodium restriction. Analysis of their interaction based on pressure-natriuresis relationship. Am J Hypertens. 1988;1:372–9. https://doi.org/10.1093/ajh/1.4.372.
Hasegawa H, Kanozawa K, Asakura J, et al. Significance of estimated salt excretion as a possible predictor of the efficacy of concomitant angiotensin receptor blocker (ARB) and low-dose thiazide in patients with ARB resistance. Hypertens Res. 2013;36:776–82. https://doi.org/10.1038/hr.2013.41.
Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481. https://doi.org/10.1038/s41440-019-0284-9.
Yoshimura H. Seasonal changes in human body fluids. Jpn J Physiol. 1958;8:165–79. https://doi.org/10.2170/jjphysiol.8.165.
Acknowledgements
We thank the participants of the study.
Funding
This work was supported by Daiichi Sankyo Co., Ltd. The funder had a role in the design and conduct of the study; in the collection and analysis of the data; and in the manuscript writing, editing, and approval to publish. The funding for the Rapid Service and Open Access fees associated with the publication was provided by Daiichi Sankyo Co., Ltd.
Medical Writing Assistance
The authors would like to thank Michelle Belanger, MD, of Edanz (www.edanz.com) for providing medical writing services, which was funded by Daiichi Sankyo Co., Ltd.
Author Contributions
All authors contributed to the substudy conception and design. Material preparation and data collection were performed by Shuichi Ichikawa and Satoru Yamakawa. Data analysis was performed by Junko Tsutsumi, and all authors contributed to the data interpretation. The first draft of the manuscript was written by Kotaro Sugimoto and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Shuichi Ichikawa received research funding from Daiichi Sankyo Co., Ltd. Junko Tsutsumi, Kotaro Sugimoto, and Satoru Yamakawa are employees of Daiichi Sankyo Co., Ltd.
Compliance with Ethics Guidelines
The protocol of this substudy was approved by the relevant institutional review boards (Supplementary Material Table S1), and all study procedures complied with the ethical principles of the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent prior to enrollment.
Data Availability
De-identified individual participant data and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo will continue to protect the privacy of our clinical study participants. Details on data sharing criteria and the procedure for requesting access are available at https://vivli.org/ourmember/daiichi-sankyo/.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ichikawa, S., Tsutsumi, J., Sugimoto, K. et al. Antihypertensive Effect of Long-Term Monotherapy with Esaxerenone in Patients with Essential Hypertension: Relationship Between Baseline Urinary Sodium Excretion and Its Antihypertensive Effect. Adv Ther 39, 4779–4791 (2022). https://doi.org/10.1007/s12325-022-02282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02282-3