Abstract
Introduction
Real-world evidence is needed to optimize pharmacotherapy for chronic obstructive pulmonary disease (COPD). The effectiveness of inhaled tiotropium/olodaterol according to baseline symptoms and previous COPD treatment and predictors of response were assessed.
Methods
This was a post hoc analysis of a 52-week post-marketing surveillance study of tiotropium/olodaterol in 1255 Japanese patients with COPD of all severities. We analyzed change in total COPD Assessment Test (CAT) score and lung function (forced expiratory volume in 1 s [FEV1] and forced vital capacity [FVC]). Patient subgroups were analyzed based on baseline CAT score (< 10 [n = 184], ≥ 10 [n = 507]) and previous COPD treatment (treatment-naive [n = 407], previously treated [n = 848], treatment with long-acting muscarinic antagonist monotherapy [n = 161]).
Results
In the CAT ≥ 10 subgroup, tiotropium/olodaterol showed statistically significant improvements in mean total CAT score (− 6.2; 95% confidence interval [CI] − 7.2, − 5.1), FEV1 (0.109 L; 95% CI 0.059, 0.159) and FVC (0.171 L; 95% CI 0.096, 0.245), which continued through Week 52. CAT score and lung function improvement were greatest in treatment-naive patients: − 7.6 (95% CI − 9.2, − 6.1) mean total CAT score, 0.177 L (95% CI 0.076, 0.279) mean FEV1 and 0.178 L (95% CI 0.036, 0.319) mean FVC. Baseline factors associated with treatment response (total CAT score improvement ≥ 2 points) were: shorter COPD duration (odds ratio [OR] 0.91; 95% CI 0.87, 0.96), total CAT score ≥ 10 (OR 3.86; 95% CI 2.46, 6.06) and treatment-naive status (OR 1.86; 95% CI 1.12, 3.07). Baseline total CAT scores ≥ 13 predicted responses to tiotropium/olodaterol in all previous COPD treatment subgroups including treatment-naive patients.
Conclusions
Tiotropium/olodaterol improved symptoms and lung function in Japanese COPD patients. Our results support the possible use of tiotropium/olodaterol in treatment-naive patients and those with total CAT scores ≥ 10.
Trail Registration
Clinicaltrials.gov Identifier for parent study: NCT02850978.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Long-acting muscarinic antagonists (LAMA) and/or inhaled long-acting β2-adrenoceptor agonists (LABA) are the backbone of maintenance pharmacotherapy for stable chronic obstructive pulmonary disease (COPD). |
COPD assessment test (CAT) score cut-points ≥ 10, cited as a criterion for COPD classification in the Global Initiative for Chronic Obstructive Lung Disease report, are not generally derived from real-world data. |
There are insufficient data on the real-world effectiveness and safety of combination therapy in treatment-naive settings, in patients with mild or severe COPD, and in those with common comorbidities such as heart failure, asthma and ischemic heart disease. |
Using data from a 52-week post-marketing surveillance study in Japanese COPD patients, this post hoc analysis sought to assess the real-world effectiveness of a fixed-dose combination of tiotropium (LAMA)/olodaterol (LABA) in subgroups defined by their baseline total CAT score and previous COPD treatment status. |
What was learned from this study? |
Tiotropium/olodaterol treatment (in ≥ 1200 cases) was associated with sustained improvements in symptoms and lung function over 52 weeks; however, these benefits were particularly evident in treatment-naive patients and in those with a total CAT score ≥ 10, supporting the classifications cited in the GOLD report and confirming the real-world effectiveness of fixed-dose tiotropium/olodaterol in subgroups of Japanese patients with COPD. |
Introduction
Bronchodilation, using an inhaled long-acting muscarinic antagonist (LAMA) and/or an inhaled long-acting β2-adrenoceptor agonist (LABA), is the backbone of maintenance pharmacotherapy for stable chronic obstructive pulmonary disease (COPD). Japanese guidelines on bronchodilator treatment for COPD [1], as well as those published by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report [2] and the American Thoracic Society (ATS) guideline [3], recommend initiating treatment with LAMA or LABA monotherapy, with subsequent ‘step up’ to combination LAMA/LABA treatment when required because of worsening clinical disease. LAMA/LABA is also recommended as an initial treatment for patients with symptoms such as a severe breathlessness or dyspnea [2, 3].
Although there is much evidence from randomized controlled trials that combination LAMA/LABA therapy is superior to monotherapy with LAMA or LABA alone [4,5,6], many questions remain. In particular, there are insufficient data on the real-world effectiveness and safety of combination therapy in the treatment-naive setting, in patients with mild COPD and in those with common comorbidities such as heart failure, asthma and ischemic heart disease. It is therefore important to undertake real-world studies of COPD treatments to assess whether the findings of clinical trials are reproduced in clinical practice. Moreover, COPD Assessment Test (CAT) score cut-offs that are cited in the GOLD report [2] are generally not derived from real-world settings, and there is a need to address this.
Tiotropium/olodaterol (Spiolto® Respimat®, Nippon Boehringer Ingelheim, Tokyo, Japan) is a once-daily, fixed-dose combination of a LAMA (tiotropium bromide, hereafter referred to as tiotropium), and a LABA (olodaterol) that is approved for the treatment of COPD in Japan and elsewhere [7, 8]. Recently, a 52-week post-marketing surveillance (PMS) study was conducted in > 1200 patients who were prescribed inhaled tiotropium/olodaterol for the treatment of COPD in Japan [9]. Here, we describe the results of a post hoc analysis of the PMS study, undertaken to address some of the knowledge gaps described above.
Methods
Objectives
We performed a post hoc analysis of data from a completed PMS study (hereafter referred to as the ‘parent study’) [9] to assess the real-world effectiveness of a fixed-dose combination of tiotropium/olodaterol in subgroups of patients defined by their baseline total CAT score and previous COPD treatment status. A secondary objective was to identify predictors of response to tiotropium/olodaterol.
Parent Study
The parent study was conducted at 199 centers throughout Japan and was registered on ClinicalTrials.gov (NCT02850978) [9, 10]. Eligibility criteria and study endpoints are listed in the online supplementary material. Observations were performed at the start of treatment (baseline) and at 4, 12, 24 and 52 weeks, or at the time of discontinuation. Because of the nature of PMS, data were extracted from medical records where available. The study was conducted by Nippon Boehringer Ingelheim Co., Ltd. (Tokyo, Japan), in accordance with Good Post-Marketing Study Practice (GPSP) regulations in Japan [11]. The study was exempt from written informed consent and institutional review board approval from each participating institution because the study was conducted in accordance with the GPSP guidelines. This article is based on a previously conducted study and does not contain any new studies with human participants performed by any of the authors. In total, 1255 patients were evaluable for effectiveness. The main results have been published previously [9] and are not described in this report.
Post Hoc Subgroup Analyses
We analyzed treatment effectiveness in four subgroups of patients: two defined by baseline total CAT score (< 10 and ≥ 10) and two defined by previous COPD treatment status (treatment-naive and previously treated). ‘Previous COPD treatment’ was defined as the use of any pulmonary medications for COPD in the 28 days before baseline and including treatments still in use at baseline. Effectiveness was also analyzed in previously treated patients who ‘stepped up’ from LAMA monotherapy to LAMA/LABA combination therapy (hereafter referred to as ‘LAMA monotherapy’ patients).
Effectiveness was evaluated as the change from baseline in total CAT scores and in lung function (forced expiratory volume in 1 s [FEV1] and forced vital capacity [FVC]). Changes from baseline in total CAT score, FEV1 and FVC were analyzed for patients with at least one set of paired data (i.e., a value at one or more time points in addition to baseline). Patients without paired data were excluded from the analyses. In addition, we calculated rates of response to tiotropium/olodaterol, with response defined as an improvement in total CAT score equal to or greater than the minimal clinically important difference (MCID; i.e., ≥ 2 points reduction) between baseline and the last observation. Lastly, patient demographics were compared between subgroups, including subgroups by severity.
Statistical Analysis
Change from baseline data in total CAT score, individual CAT item scores, FEV1, FEV1 percent predicted and FVC were analyzed using descriptive statistics, including mean, standard deviation and 95% confidence intervals [CIs]. Statistical comparisons of demographic variables between subgroups were performed using Student’s t-test for continuous variables (2 groups), analysis of variance for continuous variables (> 2 groups) and chi-squared test for categorical variables.
Univariate and multivariate logistic regression analyses were performed to identify predictors of response to tiotropium/olodaterol. In the univariate analyses, odds ratios (with 95% CIs and P values) were calculated for subgroups of patients defined by age, sex, bodyweight, body mass index (BMI), smoking history, duration of COPD, baseline total CAT score, lung function, COPD severity, COPD treatment history and the presence (or a history) of bronchial asthma. Several of these variables (age, sex, BMI, duration of COPD, baseline total CAT score, baseline FVC and COPD treatment history) were explored further in multivariate analyses.
To determine the total CAT score at baseline with the greatest predictive value for response/non-response (as defined above), receiver-operating characteristic (ROC) curves were constructed by plotting the true-positive ratio (TPR; sensitivity) on the y-axis and false-positive ratio (FPR; 1—specificity) on the x-axis.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics
Of the 1255 patients in the effectiveness analysis, baseline CAT scores were available for 691 (55.1%; 184 patients in the CAT < 10 subgroup and 507 patients in the CAT ≥ 10 subgroup). Four hundred seven patients (32.4%) were COPD treatment–naive, and 848 patients (67.6%) had been previously treated. Of the previously treated patients, 161 had received LAMA monotherapy.
Baseline demographics, disease and treatment information by subgroup are shown in Tables S1 and S2 in the online supplementary material. Additionally, Table S3 in the online supplementary material shows baseline data for subgroups of patients defined by COPD severity (mild, moderate, severe or very severe) at baseline; outcomes in these subgroups have been presented previously [9].
The mean age was 73.3 ± 9.0 years, 83.9% of patients were male, and 20.2% had mild COPD. The subgroups were generally comparable with respect to mean age, age category and sex (Tables S1 and S2). Lung function was worse in patients with higher versus lower total CAT scores at baseline: mean FEV1 was significantly lower in the CAT ≥ 10 subgroup than in the CAT < 10 subgroup (58.285% vs 66.687% predicted, respectively; P < 0.001; Table S1). Patients in the treatment-naive subgroup were less likely to be classed as having severe COPD than those in the previously treated subgroup (20.4% vs 25.4%; P = 0.003; Table S2). However, they had higher mean total CAT scores than those in the previously treated subgroup (16.9 vs 15.0; P = 0.008). Compared with the treatment-naive subgroup, LAMA monotherapy patients had a lower baseline mean total CAT score (14.4 vs 16.9; P = 0.015).
Effectiveness by CAT Score at Baseline
Changes in Total CAT Score
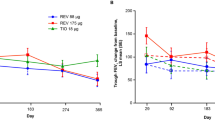
Changes in mean total CAT score for subgroups defined by total CAT score at baseline are shown in Fig. 1a and Table 1. In the CAT < 10 subgroup, mean total CAT scores were slightly worse (i.e., higher) during the study than at baseline, but differences from baseline were not statistically significant except at Week 24 (Fig. 1a). However, in the CAT ≥ 10 subgroup, tiotropium/olodaterol was associated with significant, sustained improvement in mean total CAT scores versus baseline from Week 4 onwards (Fig. 1a). In this subgroup, the mean change from baseline in total CAT score was − 4.6 (95% CI − 5.2, − 3.9) at Week 4, improving to − 6.2 (95% CI − 7.2, − 5.1) at Week 52. Changes in score for individual items of the CAT questionnaire were generally consistent with changes in total score for both subgroups (see Table S4). In the CAT < 10 subgroup, mean change from baseline for all individual item scores was ± 0.3 at all time points. The only significant change from baseline was in the breathlessness score at Week 4 (− 0.3; 95% CI − 0.5, − 0.1). In the CAT ≥ 10 subgroup, however, significant reductions (improvements) in scores for all individual items were achieved by Week 4, and the magnitude of change from baseline either increased or was sustained at later time points. The greatest reductions in score at Week 52 were seen for breathlessness (change from baseline of − 1.1) and chest tightness (− 0.9).
Change from baseline in a mean total COPD Assessment Test (CAT) score; b forced expiratory volume in 1 s (FEV1), expressed as a percentage of the predicted value; c FEV1, expressed in liters, for subgroups defined by baseline total CAT score. Error bars represent 95% confidence intervals. COPD chronic obstructive pulmonary disease
Changes in Lung Function
Mean FEV1 values were higher than baseline throughout the study in both CAT score subgroups (Fig. 1b and c, and Table 1). The change from baseline in FEV1 was similar and statistically significant for both subgroups at Week 4 (0.118 L in the CAT < 10 subgroup and 0.121 L in the CAT ≥ 10 subgroup) and Week 12 (0.104 L in the CAT < 10 subgroup and 0.109 L in the CAT ≥ 10 subgroup) and remained significant at later time points in the CAT ≥ 10 subgroup.
Changes from baseline in FVC are shown in Table 1. In the CAT ≥ 10 subgroup, statistically significant, sustained improvements in mean FVC of 0.129–0.171 L from baseline were observed between Weeks 4 and 52. In the CAT < 10 subgroup, FVC also improved from baseline, but to a lesser extent than in the CAT ≥ 10 subgroup.
Effectiveness by Previous Treatment Status
Changes in Total CAT Score
Changes in mean total CAT score, according to previous COPD treatment status, are shown in Table 1. Tiotropium/olodaterol treatment was associated with significant and sustained reductions in total CAT score, with the magnitude of change being larger in the treatment-naive subgroup (− 4.4 at Week 4, increasing to − 7.6 at Week 52) than in the previously treated subgroup (− 2.8 at Week 4, increasing to − 3.1 at Week 52) at all time points.
In patients who had previously received LAMA monotherapy, improvements in CAT score were significant versus baseline at each time point, indicating benefit of transitioning from LAMA monotherapy to LAMA/LABA combination therapy. Total CAT scores in these patients improved by − 4.1 at Week 4 and remained stable thereafter (mean change from baseline − 3.6 at Week 52).
Mean scores for individual items of the CAT showed patterns of change (see Table S5) that were similar to changes in total scores for these subgroups. Previously treated patients had reductions in scores for cough, phlegm, chest tightness and breathlessness that were statistically significant, compared with baseline, at all time points. As for total CAT score, changes from baseline in scores for individual items were numerically greater for treatment-naive versus previously treated patients. Regarding sleep scores, previously treated patients did not show significant reductions but treatment-naive patients did at all time points.
Changes in Lung Function
Mean FEV1 values were higher than baseline throughout the study in both subgroups defined by previous COPD treatment status and in LAMA monotherapy patients (Table 1). Changes from baseline in FEV1 at each time point were greatest in the treatment-naive subgroup, except at Week 24 when the change from baseline was similar to that in previously treated patients. In LAMA monotherapy patients, the change from baseline in FEV1 was not significant at Week 12 and Week 24, and in the treatment-naive subgroup, the change from baseline was not significant at Week 24. All other changes from baseline were statistically significant.
Changes from baseline in FVC (Table 1) were broadly consistent with changes in FEV1, and indicated improvement in lung function over the course of the study.
Response Analyses
In univariate subgroup analyses, odds ratios were highest in patients who were treatment-naive, had a baseline CAT score ≥ 10, were aged < 65 years or had a duration of COPD below the median value of 2.5 years. We observed significant effects of baseline age, COPD duration, total CAT score, FVC and previous COPD treatment (yes vs no) on response rates using ‘last observation’ data (Fig. 2). The results of the multivariate analyses are shown in Fig. 3. Shorter duration of COPD, baseline total CAT score ≥ 10 and treatment-naive status were positively associated with response; baseline total CAT score was the strongest predictor of response (or non-response) at all time points.
Univariate analysis of response rates to tiotropium/olodaterol. Response was defined as an improvement of ≥ 2 in total CAT score between baseline and last observation. Data are shown as odds ratios with 95% confidence intervals (CIs). aMedian bodyweight at baseline: 58.0 kg. bMedian pack-years at baseline: 48.0. cMedian COPD duration at baseline: 2.5 years. dMedian total CAT score at baseline: 15.0. eMedian FEV1 at baseline: 1.425 l. fMedian FVC at baseline: 2.67 l. gMedian FEV1 (% predicted) at baseline: 59.1%. CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, ICS inhaled corticosteroid
Multivariate analysis of response rates to tiotropium/olodaterol. Response was defined as an improvement of ≥ 2 in total CAT score between baseline and last observation. Data are shown as odds ratios with 95% confidence intervals (CI). CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, FVC forced vital capacity, ICS inhaled corticosteroid
The results of the ROC analysis are shown in Fig. 4. A baseline CAT score of ≥ 13 was predictive of response to tiotropium/olodaterol at last observation in all previous COPD treatment groups. The optimal total CAT score cut-offs were associated with sensitivities of 56% and specificities of 67%.
Receiver-operating characteristic (ROC) curve of baseline CAT score as a predictor of response in all previous COPD treatment groups (n = 634; 384 responders and 250 non-responders). Response was defined as an improvement of ≥ 2 in total CAT score between baseline and the last observation. The solid red line indicates the point on the curve that is closest to the top-left of the graph and corresponds to the baseline CAT score cut-off value that predicts response/non-response with greatest sensitivity and specificity (indicated by dashed black lines). CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease
Discussion
This post hoc analysis of data from a large (> 1200 patients) PMS study provides valuable insights into the real-world effectiveness of tiotropium/olodaterol in Japanese people with COPD. Our study population was representative of the patients seen in clinical practice in Japan and had a broad spectrum of comorbidities in all COPD severity subgroups, including patients who were treatment naive. Overall, 20% of patients had mild COPD, in which there is a general lack of evidence, and 32% were treatment-naive at baseline.
The findings, in the approximately 600 patients for whom baseline CAT score and lung function data were available, were consistent with the effectiveness results of the parent study [9]. Total CAT scores showed significant, sustained and clinically meaningful (i.e., > MCID) improvements from baseline in patients with initial total CAT scores ≥ 10. Consistent with the observed changes in total CAT score, lung function (FEV1 and FVC) also showed significant and sustained improvement from baseline in the CAT ≥ 10 subgroup. Furthermore, this subgroup showed significant reductions in all eight individual items of the CAT.
Patients with total CAT scores < 10 at baseline, while having an increase in total CAT score at Week 24, had similar total CAT scores at Week 52 compared with baseline. Although the change of magnitude in CAT, and also FVC, in this subgroup over the entire study period was admittedly small, FEV1 had improved as much as in those with a baseline CAT score ≥ 10 at Week 12. This effect on FEV1 did not persist to Week 52, but the effect earlier in the study does indicate that some benefit from the therapeutic intervention was apparent even in these patients with low baseline CAT scores. In addition, the breathlessness item of the CAT was reduced at all time points. While the improvement in CAT score in patients with a CAT score < 10 did not exceed the MCID, the baseline mean total CAT score in this subgroup was 5.4, describing a population with mild symptoms and a relatively good overall health status. Patients with better health status and fewer symptoms might be less motivated to adhere to treatment. Due to the observational nature of the PMS study, we did not systematically assess adherence and are therefore unable to determine whether suboptimal adherence contributed to the results in this subgroup. As a whole, these findings suggest that tiotropium/olodaterol is beneficial in the real-world setting and that the perceived benefit of treatment is higher when CAT is ≥ 10. These findings should help physicians make decisions about COPD treatment.
Tiotropium/olodaterol was associated with significant improvements in total CAT scores at all time points in both treatment-naive and previously treated patients. The baseline total CAT score was significantly higher in the treatment-naive subgroup than in the previously treated subgroup, but the greater improvement in total CAT score in the former group could indicate that treatment has a greater impact in treatment-naive patients. Patients who ‘stepped up’ from LAMA monotherapy to tiotropium/olodaterol also had significant improvements in total CAT score that were evident at Week 4 and sustained through Week 52. In particular, the ‘sleep’ and ‘confident leaving home’ items were reduced significantly at all time points in treatment-naive patients, and the magnitude of change was greater than that in the previously treated patients. The effects seen in our analysis are consistent with a comparison of LAMA/LABA versus LAMA monotherapy in Japanese patients with untreated COPD, which showed a significant improvement in FEV1, inspiratory capacity and CAT scores with the combination compared with LAMA monotherapy and that the CAT items of ‘sleep’ and ‘confident leaving home’ were significantly improved compared with LAMA monotherapy [12]. The improvement in inspiratory capacity may have resulted in an improvement in these CAT items.
As in the subgroup of patients with baseline total CAT scores ≥ 10, treatment-naive patients experienced additional incremental improvements in total CAT score with continued treatment beyond Week 4. However, these improvements in CAT score were not accompanied by corresponding sustained improvements in lung function: at Week 24, there was a temporary decrease in both FEV1 and FVC in this subgroup (although both parameters were still improved versus baseline). Although the cause of this temporary decrease is unknown, COPD is a heterogeneous disease and unexplained variations in lung function may occur. Furthermore, since CAT provides an assessment of subjective symptoms, it does not necessarily correlate with FEV1 and FVC, which are objective assessment tools; therefore, the changes in FEV1 and FVC will not necessarily be consistent between the CAT groups or treatment groups. Also, because the PMS study was non-interventional and observational, it was not specified that CAT and respiratory function tests should be performed at every time point (i.e., point-to-point data are not available for every patient). Since the assessments were performed at the discretion of the physician, the possibility of unstable patients with more severe symptoms at Week 24 cannot be ruled out.
Interestingly, there was no clear and consistent difference between the previous treatment subgroups with regards to FVC, but there was a better response in FEV1 in the treatment-naive patients. On the other hand, although there was a much better response according to FVC in the patients with CAT score of ≥ 10 compared with those with a score of < 10, there was no consistent difference between the baseline CAT groups according to FEV1. A reason for these contradictory results could be a response of the airways to treatment, as demonstrated by changes in FEV1, which may have been easier to demonstrate in the patients who were treatment-naive. These patients appeared to have hyperinflation (treatment-naive patients: mean baseline CAT score, 17.1 ± 8.6; previously treated patients: mean baseline CAT score, 14.9 ± 8.5). However, there was no difference in FVC in previous COPD treatment subgroups, suggesting that hyperinflation was not fully improved even if the previously treated patients had received COPD treatments. A CAT score of < 10 suggests little to no lung hyperinflation; therefore, changes in FVC (an indirect measure of hyperinflation) may be less likely to occur with treatment in patients with a low CAT score. The subgroup with a CAT score ≥ 10 responded to the treatment intervention, as evidenced by a trend in FEV1. It should be noted that the percentage of treatment-naive patients with a baseline CAT score of ≥ 10 was similar to the percentage with a score of < 10 (29.0% and 23.4%, respectively).
In both univariate and multivariate analyses, we found that a shorter duration of COPD, a higher total CAT score (≥ 10) and treatment-naive status were associated with a significantly greater likelihood of response to tiotropium/olodaterol, defined as an improvement in total CAT score of ≥ 2 (the MCID) from baseline at last observation. The effectiveness of tiotropium/olodaterol appears to be greater earlier in the course of COPD in patients who have total CAT scores of ≥ 10 than for those patients with a total CAT score of < 10, including those who are not yet receiving maintenance treatment.
Analysis of ROC curves showed that the evident total CAT score threshold for predicting response/non-response to tiotropium/olodaterol (defined according to the CAT score MCID) was 13 for all previous COPD treatment groups. Our results are in line with the current GOLD report [2], in which treatment recommendations differ for patients with total CAT scores above or below 10. Although data on exacerbations were not available in this study, tiotropium/olodaterol would provide potential benefits in patients with CAT ≥ 10 in GOLD groups B and D for initial pharmacology therapy, even though their CAT scores are less than 20. However, it should be noted that the baseline mean CAT total scores were 16.6 in the severe and 20.7 in very severe COPD groups, respectively.
In a randomized controlled trial of 80 treatment-naive Japanese patients (> 80% GOLD 2), tiotropium/olodaterol was associated with significantly greater improvements in FEV1 and dyspnea than monotherapy [13]. In addition, post hoc analyses of phase III trials in patients with moderate-to-severe COPD have found that tiotropium/olodaterol improves lung function, symptoms and health status, and delays disease progression, to a greater extent than tiotropium monotherapy [5, 6, 14].
There is also real-world evidence that, among patients prescribed tiotropium/olodaterol, there is a greater probability of improvement in symptoms and health status (measured using the Clinical COPD Questionnaire [CCQ]) in treatment-naive patients than in previously treated patients [15].
Our study has several limitations that should be considered when interpreting the findings. As PMS studies are non-interventional and observational, there were no restrictions on the use of concomitant medications during the study period. Furthermore, although switching to or initiating a LAMA/LABA combination is recommended for patients with severe symptoms or who are still symptomatic despite LAMA monotherapy, the purely observational nature of the PMS study meant that physicians made their own decisions regarding their patients’ need for LAMA/LABA therapy, and their motivations for prescribing the combination were not recorded. As such, our results may need to be confirmed in analyses that, unlike PMS studies, include more rigorous drug administration protocols and a comparative control arm. Additionally, CAT scores and lung function measurements were not collected from all patients according to a predetermined schedule; consequently, only available data at each time point could be analyzed. The use of different formulae to calculate FEV1 percent predicted, due to differences in the type of spirometer used at different study centers, could have affected our findings. We did not formally assess adherence or inhaler technique and therefore cannot evaluate the possible contribution of suboptimal drug exposure to our results; this may be particularly relevant in patients with a low disease burden, and in patients with comorbidities that interfere with inhaler use or technique. We did not specifically assess safety in the subgroup analyses, and therefore cannot draw any conclusions about the comparative safety of tiotropium/olodaterol in subgroups defined by baseline total CAT score or previous COPD treatment status; however, no safety concerns were identified in the main PMS analysis [9]. Lastly, we did not perform statistical calculations to determine the required sample size for the subgroup analyses. Our results are therefore partially descriptive, and significance is inferred from 95% CIs.
As mentioned above, the outcomes data used in our analyses were not mandatorily obtained in accordance with a protocol. In real-world clinical practice, patients who are in good health may not undergo regular evaluation, while those in poor health are more likely to be assessed; this may represent a further source of bias in our research.
Conclusion
This large PMS study demonstrates the real-world effectiveness of fixed-dose tiotropium/olodaterol in subgroups of Japanese patients with COPD. Treatment was associated with sustained improvements in symptoms and lung function over 52 weeks. The benefits of tiotropium/olodaterol appeared to be evident in treatment-naive patients and in those with a total CAT score ≥ 10.
References
Japanese Respiratory Society. Guidelines for COPD diagnosis and treatment 2022 (6th edition) [in Japanese]. 2022. https://www.jrs.or.jp/publication/jrs_guidelines/20220512084311.html. Accessed 1 Aug 2022.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report. 2021. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed 1 Apr 2021.
Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e56–69.
Buhl R, Singh D, de la Hoz A, Xue W, Ferguson GT. Benefits of tiotropium/olodaterol compared with tiotropium in patients with COPD receiving only LAMA at baseline: pooled analysis of the TONADO® and OTEMTO® studies. Adv Ther. 2020;37:3485–99.
Rabe KF, Chalmers JD, Miravitlles M, et al. Tiotropium/olodaterol delays clinically important deterioration compared with tiotropium monotherapy in patients with early COPD: a post hoc analysis of the TONADO® trials. Adv Ther. 2021;38:579–93.
Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO® studies. Respir Res. 2016;17:73.
Ministry of Health, Labour and Welfare. Report on the deliberation results. Spiolto respimat. 2015. https://www.pmda.go.jp/files/000216005.pdf. Accessed 1 Apr 2021.
Blair HA. Tiotropium/olodaterol: a review in COPD. Drugs. 2019;79:997–1008.
Nakamura S, Miyazaki A, Ikeda R, Kinoshita Y. Safety and efficacy of tiotropium/olodaterol fixed-dose combination in Japanese patients with chronic obstructive pulmonary disease: a 52-week post-marketing surveillance (in Japanese). Ther Res. 2020;41:195–221.
ClinicalTrials.gov. Long-term use of spiolto respimat in Japanese patients with chronic obstructive pulmonary disease. https://clinicaltrials.gov/ct2/show/NCT02850978. Accessed 2 Apr 2020.
Japanese Pharmaceutical Manufacturers Association. Post-marketing surveillance of drugs. 2004. http://www.jpma.or.jp/english/parj/pdf/2015_ch04.pdf. Accessed 1 Apr 2021.
Yamada H, Hida N, Hizawa N. Effects of a single long-acting muscarinic antagonist agent and a long-acting muscarinic antagonist/long-acting beta2-adrenoceptor agonist combination on lung function and symptoms in untreated COPD patients in Japan. Int J Chron Obstr Pulm Dis. 2018;13:3141–7.
Takahashi K, Uchida M, Kato G, et al. First-line treatment with tiotropium/olodaterol improves physical activity in patients with treatment-naïve chronic obstructive pulmonary disease. Int J Chron Obstr Pulm Dis. 2020;15:2115–26.
Buhl R, de la Hoz A, Xue W, Singh D, Ferguson GT. Efficacy of tiotropium/olodaterol compared with tiotropium as a first-line maintenance treatment in patients with COPD who are naïve to LAMA, LABA and ICS: pooled analysis of four clinical trials. Adv Ther. 2020;37:4175–89.
Valipour A, Avdeev S, Barczyk A, et al. Therapeutic success of tiotropium/olodaterol, measured using the clinical COPD questionnaire (CCQ), in routine clinical practice: a multinational non-interventional study. Int J Chron Obstr Pulm Dis. 2021;16:615–28.
Acknowledgements
Funding
Sponsorship for this study and the journal’s Rapid Service Fee were funded by Nippon Boehringer Ingelheim Co., Ltd, the manufacturer of Spiolto® Respimat® (tiotropium/olodaterol).
Medical writing assistance
Medical writing support, funded by Nippon Boehringer Ingelheim, was provided by Richard Crampton of in Science Communications, Springer Healthcare.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed to the study conception and design, interpretation of study results, and in the drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Disclosures
Atsuyasu Sato has received grants and personal fees from Nippon Boehringer Ingelheim; grants from Bayer; personal fees from AstraZeneca; and grants from LUCA Science Inc. Ai Miyazaki and Shuhei Nakamura are employees of Nippon Boehringer Ingelheim Co. Ltd.
Compliance with ethics guidelines
This study was conducted by Nippon Boehringer Ingelheim Co., Ltd. (Tokyo, Japan), in accordance with Good Post-Marketing Study Practice (GPSP) regulations in Japan. The study was exempt from requirement for written informed consent and from institutional review board approval from each participating institution because the study was conducted in accordance with the GPSP guidelines. This article is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Data availability
The study data are available to be shared upon reasonable request. Researchers should submit enquiries via https://trials.boehringer-ingelheim.com/.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sato, A., Miyazaki, A. & Nakamura, S. Effectiveness of Tiotropium/Olodaterol in the Real World: A Post Hoc Subgroup Analysis After the First Year of Use. Adv Ther 39, 4692–4706 (2022). https://doi.org/10.1007/s12325-022-02268-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02268-1