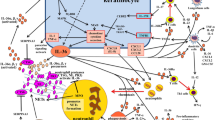
Abstract
Autoimmune inflammatory diseases are primarily characterized by deregulated expression of cytokines, which drive pathogenesis of these diseases. A number of approved and experimental therapies utilize monoclonal antibodies against cytokine proteins. Cytokines can be classified into different families including the interleukins, which are secreted and act on leukocytes, the tumor necrosis factor (TNF) family, as well as chemokine proteins. In this review article, we focus on the interleukin family of cytokines, of which 39 members have been identified to this date. We outline the role of each of these interleukins in the immune system, and various dermatological inflammatory diseases with a focused discussion on the pathogenesis of psoriasis and atopic dermatitis. In addition, we describe the roles of various interleukins in psychiatric, cardiovascular, and gastrointestinal comorbidities. Finally, we review clinical efficacy and safety data from emerging late-phase anti-interleukin therapies under development for psoriasis and atopic dermatitis. Collectively, additional fundamental and clinical research remains necessary to fully elucidate the roles of various interleukin proteins in the pathogenesis of inflammatory dermatologic diseases, and treatment outcomes in patients.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Murphy K, Weaver C. Janeway’s Immunobiology. 9th edition. Garland Science.
Revised nomenclature for antigen-nonspecific T cell proliferation and helper factors. J Immunol. 1979;123:2928–9.
Nomenclature for secreted regulatory proteins of the immune system (interleukins). WHO-IUIS Nomenclature Subcommittee on Interleukin Designation. Bull World Health Organ. 1991;69:483–6.
Nomenclature for secreted regulatory proteins of the immune system (interleukins): update. IUIS/WHO Standing Committee on Interleukin Designation. Bull World Health Organ. 1997;75:175.
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010.
Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genom. 2010;5:30–55.
Hymowitz SG, Filvaroff EH, Yin J, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–41.
Coondoo A. Cytokines in dermatology—a basic overview. Indian J Dermatol. 2011;56:368–74.
Dinarello CA. Historical review of cytokines. Eur J Immunol. 2007;37:S34-45.
Jia H, Liu J, Han B. Reviews of interleukin-37: functions, receptors, and roles in diseases. BioMed Res Int. 2018;2018: e3058640.
Lin H, Ho AS, Haley-Vicente D, Zhang J, Bernal-Fussell J, Pace AM, et al. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member*. J Biol Chem. 2001;276:20597–602.
Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–63.
Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56.
Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–40.
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509.
Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–20.
Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432–7.
Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei X-Y, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–8.
Viguier M, Guigue P, Pagès C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66–7.
Hüffmeier U, Wätzold M, Mohr J, Schön MP, Mössner R. Successful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutations. Br J Dermatol. 2014;170:202–4.
Ganesan R, Raymond EL, Mennerich D, Woska JR, Caviness G, Grimaldi C, et al. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs. 2017;9:1143–54.
Tauber M, Viguier M, Le Gall C, Smahi A, Bachelez H. Is it relevant to use an interleukin-1-inhibiting strategy for the treatment of patients with deficiency of interleukin-36 receptor antagonist? Br J Dermatol. 2014;170:1198–9.
Bachelez H, Choon S-E, Marrakchi S, Burden AD, Tsai T-F, Morita A, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981–3.
EMA validates spesolimab marketing authorization application in GPP | Press [Internet]. boehringer-ingelheim.com [cited 2021 Nov 14]. https://www.boehringer-ingelheim.com/press-release/ema-filing-acceptance-and-validation-spesolimab.
Reich A, Barker J, Pink A, Reynolds NJ, Griffiths CEM, Kalapanda R, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody, in the treatment of generalized pustular psoriasis: results from a phase 2 trial. 2021. p. 1–15.
Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. PNAS Natl Acad Sci. 1989;86:6367–71.
Palmou-Fontana N, Gaviño JAS, McGonagle D, García-Martinez E, Martín LIO. Tocilizumab-induced psoriasiform rash in rheumatoid arthritis. DRM. 2014;228:311–3.
Laurent S, Le Parc J-M, Clérici T, Bréban M, Mahé E. Onset of psoriasis following treatment with tocilizumab. Br J Dermatol. 2010;163:1364–5.
Wendling D, Letho-Gyselinck H, Guillot X, Prati C. Psoriasis onset with tocilizumab treatment for rheumatoid arthritis. J Rheumatol. 2012;39:657.
Fritz Y, Klenotic PA, Swindell WR, Yin ZQ, Groft SG, Zhang L, et al. Induction of alternative proinflammatory cytokines accounts for sustained psoriasiform skin inflammation in IL-17C+IL-6KO mice. J Invest Dermatol. 2017;137:696–705.
Blauvelt A. IL-6 differs from TNF-α: unpredicted clinical effects caused by IL-6 blockade in psoriasis. J Invest Dermatol. 2017;137:541–2.
Takematsu H, Tagami H. Quantification of chemotactic peptides (C5a anaphylatoxin and IL-8) in psoriatic lesional skin. Arch Dermatol. 1993;129:74–80.
Duan H, Koga T, Kohda F, Hara H, Urabe K, Furue M. Interleukin-8-positive neutrophils in psoriasis. J Dermatol Sci. 2001;26:119–24.
Trepicchio WL, Ozawa M, Walters IB, Kikuchi T, Gilleaudeau P, Bliss JL, et al. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104:1527–37.
Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, et al. Cutting edge: a critical functional role for IL-23 in psoriasis. J Immunol. 2010;185:5688–91.
Kulig P, Musiol S, Freiberger SN, Schreiner B, Gyülveszi G, Russo G, et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016;7:13466.
Diels J, Thilakarathne P, Cameron C, McElligott S, Schubert A, Puig L. Adjusted treatment COMPArisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: the COMPASS analysis. Br J Dermatol. 2020;183:276–84.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour J-P, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551–60.
Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11.
Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55:379–90.
Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–55.
Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–502.
Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142–52.
Papp KA, Weinberg MA, Morris A, Reich K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet. 2021;397:1564–75.
Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–11.
Li H-H, Lin Y-C, Chen P-J, Hsiao C-H, Lee JY-Y, Chen W-C, et al. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol. 2005;153:591–5.
Wei C-C, Chen W-Y, Wang Y-C, Chen P-J, Lee JY-Y, Wong T-W, et al. Detection of IL-20 and its receptors on psoriatic skin. Clin Immunol. 2005;117:65–72.
Kunz S, Wolk K, Witte E, Witte K, Doecke W-D, Volk H-D, et al. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004.
Lebre MC, Jonckheere CL, Kraan MC, van Kuijk AW, Bos JD, de Rie M, et al. Expression of IL-20 in synovium and lesional skin of patients with psoriatic arthritis: differential response to alefacept treatment. Arthritis Res Ther. 2012;14:R200.
Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15:1013–5.
Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard F-X, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–15.
Wolk K, Witte E, Wallace E, Döcke W-D, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23.
Pfizer. An ascending multiple dose study of the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical efficacy of ILV-094 administered subcutaneously or intravenously to subjects with psoriasis [Internet]. clinicaltrials.gov; 2011 Mar. Report No.: NCT00563524. https://clinicaltrials.gov/ct2/show/NCT00563524.
Wang F, Smith N, Maier L, Xia W, Hammerberg C, Chubb H, et al. Etanercept suppresses regenerative hyperplasia in psoriasis by acutely downregulating epidermal expression of interleukin (IL)-19, IL-20 and IL-24. Br J Dermatol. 2012;167:92–102.
Xu M, Lu H, Lee Y-H, Wu Y, Liu K, Shi Y, et al. An interleukin-25-mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787-798.e4.
Itoh T, Hatano R, Komiya E, Otsuka H, Narita Y, Aune TM, et al. Biological effects of IL-26 on T cell-mediated skin inflammation, including psoriasis. J Invest Dermatol. 2019;139:878–89.
Andrys C, Borska L, Pohl D, Fiala Z, Hamakova K, Krejsek J. Angiogenic activity in patients with psoriasis is significantly decreased by Goeckerman’s therapy. Arch Dermatol Res. 2007;298:479–83.
Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M, et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130:1034–9.
Omar NS, Long X, Xian J, Afewerky HK, Hussain SG, Peng X. Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: a case-control study. J Int Med Res. 2021;49:3000605211004039.
Mitsui A, Tada Y, Takahashi T, Shibata S, Kamata M, Miyagaki T, et al. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol. 2016;41:183–9.
Wolk K, Witte K, Witte E, Raftery M, Kokolakis G, Philipp S, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci Transl Med. 2013;5: 204ra129.
Witte E, Kokolakis G, Witte K, Warszawska K, Friedrich M, Christou D, et al. Interleukin-29 induces epithelial production of CXCR3A ligands and T-cell infiltration. J Mol Med (Berl). 2016;94:391–400.
Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405.
Kempuraj D, Conti P, Vasiadi M, Alysandratos K-D, Tagen M, Kalogeromitros D, et al. IL-32 is increased along with tryptase in lesional psoriatic skin and is up-regulated by substance P in human mast cells. Eur J Dermatol. 2010;20:865–7.
Li J, Liu L, Rui W, Li X, Xuan D, Zheng S, et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology. 2017;233:37–46.
Teng X, Hu Z, Wei X, Wang Z, Guan T, Liu N, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol. 2014;192:1815–23.
Mercurio L, Morelli M, Scarponi C, Eisenmesser EZ, Doti N, Pagnanelli G, et al. IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 2018;9:1–13.
Ständer S. Atopic dermatitis. N Engl J Med. 2021;384:1136–43.
Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40:84–92.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7.
Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5.
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS ONE. 2011;6: e22029.
Ma L, Xue H-B, Guan X-H, Shu C-M, Zhang J-H, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25–31.
Toda M, Leung DYM, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–81.
Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244-1252.e2.
Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–7.
Arai I, Tsuji M, Takeda H, Akiyama N, Saito S. A single dose of interleukin-31 (IL-31) causes continuous itch-associated scratching behaviour in mice. Exp Dermatol. 2013;22:669–71.
Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J Am Acad Dermatol. 2014;70:882–8.
Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921–6.
Lilly’s lebrikizumab significantly improved skin clearance and itch in people with moderate-to-severe atopic dermatitis in two Phase 3 trials | Eli Lilly and Company [Internet]. [cited 2021 Nov 15]. https://investor.lilly.com/news-releases/news-release-details/lillys-lebrikizumab-significantly-improved-skin-clearance-and.
Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour J-P, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184:437–49.
Kabashima K, Matsumura T, Komazaki H, Kawashima M, Nemolizumab-JP01 Study Group. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383:141–50.
Febbraio MA. Role of interleukins in obesity: implications for metabolic disease. Trends Endocrinol Metab. 2014;25:312–9.
Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1–receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26.
Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, c-reactive protein, interleukin-6, and fibrinogen. Circulation. 2012;126:2739–48.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71:2392–401.
Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3.
Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70.
Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–34.
Liu Z, Yadav PK, Xu X, Su J, Chen C, Tang M, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol. 2011;89:597–606.
Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60.
Ferreira BIRC, Abreu JLPDC, Reis JPGD, Figueiredo AMDC. Psoriasis and associated psychiatric disorders. J Clin Aesthet Dermatol. 2016;9:36–43.
Heller MM, Lee ES, Koo JY. Stress as an influencing factor in psoriasis. Skin Ther Lett. 2011;16:1–4.
Reich A, Hrehorów E, Szepietowski JC. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm Venereol. 2010;90:257–63.
Janković S, Raznatović M, Marinković J, Maksimović N, Janković J, Djikanović B. Relevance of psychosomatic factors in psoriasis: a case-control study. Acta Derm Venereol. 2009;89:364–8.
Devrimci-Ozguven H, Kundakci TN, Kumbasar H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2000;14:267–71.
Mizara A, Papadopoulos L, McBride SR. Core beliefs and psychological distress in patients with psoriasis and atopic eczema attending secondary care: the role of schemas in chronic skin disease. Br J Dermatol. 2012;166:986–93.
Garg A, Chren MM, Sands LP, Matsui MS, Marenus KD, Feingold KR, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–9.
Arnetz BB, Fjellner B, Eneroth P, Kallner A. Stress and psoriasis: psychoendocrine and metabolic reactions in psoriatic patients during standardized stressor exposure. Psychosom Med. 1985;47:528–41.
Buske-Kirschbaum A, Ebrecht M, Kern S, Hellhammer DH. Endocrine stress responses in TH1-mediated chronic inflammatory skin disease (psoriasis vulgaris)—do they parallel stress-induced endocrine changes in TH2-mediated inflammatory dermatoses (atopic dermatitis)? Psychoneuroendocrinology. 2006;31:439–46.
Ganança L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology. 2016;63:296–310.
Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30.
Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–33.
Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–8.
Saraceno R, Kleyn CE, Terenghi G, Griffiths CEM. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876–82.
Turka LA, Goodman RE, Rutkowski JL, Sima AA, Merry A, Mitra RS, et al. Interleukin 12: a potential link between nerve cells and the immune response in inflammatory disorders. Mol Med. 1995;1:690–9.
Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999–2009.
Maddison DC, Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol. 2015;40:134–41.
Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–8.
Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Annu Rev Med. 2014;65:223–44.
Witte-Händel E, Wolk K, Tsaousi A, Irmer ML, Mößner R, Shomroni O, et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139:1294–305.
Zhou J, Luo Q, Cheng Y, Wen X, Liu J. An update on genetic basis of generalized pustular psoriasis (review). Int J Mol Med. 2021;47:1–12.
Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52.
Dusser P, Koné-Paut I. IL-1 inhibition may have an important role in treating refractory Kawasaki disease. Front Pharmacol. 2017;8:1–8.
Aouba A, Georgin-Lavialle S, Pagnoux C, Martin Silva N, Renand A, Galateau-Salle F, et al. Rationale and efficacy of interleukin-1 targeting in Erdheim–Chester disease. Blood. 2010;116:4070–6.
Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748–51.
Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11-14.
Chiricozzi A, Maurelli M, Peris K, Girolomoni G. Targeting IL-4 for the treatment of atopic dermatitis. Immunotargets Ther. 2020;9:151–6.
Salmon-Ehr V, Serpier H, Nawrocki B, Gillery P, Clavel C, Kalis B, et al. Expression of interleukin-4 in scleroderma skin specimens and scleroderma fibroblast cultures. Potential role in fibrosis. Arch Dermatol. 1996;132:802–6.
Schmidt E, Bastian B, Dummer R, Tony HP, Bröcker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288:353–7.
Abdat R, Waldman RA, de Bedout V, Czernik A, Mcleod M, King B, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83:46–52.
Roufosse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne). 2018;5:1–27.
Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–32.
Nakajima S, Tie D, Nomura T, Kabashima K. Novel pathogenesis of atopic dermatitis from the view of cytokines in mice and humans. Cytokine. 2021;148: 155664.
Xu H, Xiao X, He Y, Zhang X, Li C, Mao Q, et al. Increased serum interleukin-6 levels in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2017;34:82–4.
Ogata A, Kumanogoh A, Tanaka T. Pathological role of interleukin-6 in psoriatic arthritis. Arthritis. 2012;2012:1–6.
Ting EY-C, Yang AC, Tsai S-J. Role of interleukin-6 in depressive disorder. Int J Mol Sci. 2020;21:1–22.
Lin Z-Q, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–21.
Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21:965–73.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34.
Yamanaka K, Clark R, Rich B, Dowgiert R, Hirahara K, Hurwitz D, et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107:2440–5.
Horev L, Unger S, Molho-Pessach V, Meir T, Maly A, Stepensky P, et al. Generalized verrucosis and HPV-3 susceptibility associated with CD4 T-cell lymphopenia caused by inherited human interleukin-7 deficiency. J Am Acad Dermatol. 2015;72:1082–4.
Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–91.
Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet. 2011;43:932–9.
Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, Bijlsma JWJ, et al. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjögren’s syndrome correlates with increased inflammation. Arthritis Rheum. 2010;62:969–77.
Herold KC, Bucktrout SL, Wang X, Bode BW, Gitelman SE, Gottlieb PA, et al. Immunomodulatory activity of humanized anti-IL-7R monoclonal antibody RN168 in subjects with type 1 diabetes. JCI Insight. 2019;4: 126054.
Ellis J, van Maurik A, Fortunato L, Gisbert S, Chen K, Schwartz A, et al. Anti-IL-7 receptor α monoclonal antibody (GSK2618960) in healthy subjects—a randomized, double-blind, placebo-controlled study. Br J Clin Pharmacol. 2019;85:304–15.
Oka M, Berking C, Nesbit M, Satyamoorthy K, Schaider H, Murphy G, et al. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Invest. 2000;80:595–604.
Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer. 2019;7:1–8.
Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–22.
Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, Fishelevich R, et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J Invest Dermatol. 2014;134:1903–11.
Gutin L, Tammaro A, Fishelevich R, Gaspari AA. Elevation of IL-9 in extreme patch test reactions suggests it is an inflammatory mediator in allergic contact dermatitis. Dermatitis. 2016;27:35–6.
Vieyra-Garcia PA, Wei T, Naym DG, Fredholm S, Fink-Puches R, Cerroni L, et al. STAT3/5-dependent IL9 overexpression contributes to neoplastic cell survival in mycosis fungoides. Clin Cancer Res. 2016;22:3328–39.
Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6:219ra8.
Weiss E, Mamelak AJ, La Morgia S, Wang B, Feliciani C, Tulli A, et al. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Acad Dermatol. 2004;50:657–75 (quiz 676–678).
Shi J, Li J, Guan H, Cai W, Bai X, Fang X, et al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS ONE. 2014;9: e98228.
Schafer S, Viswanathan S, Widjaja AA, Lim W-W, Moreno-Moral A, DeLaughter DM, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–5.
Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. 2006;5:825–32.
Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52.
Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254–60.
Biedermann T, Lametschwandtner G, Tangemann K, Kund J, Hinteregger S, Carballido-Perrig N, et al. IL-12 instructs skin homing of human Th2 cells. J Immunol. 2006;177:3763–70.
Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31.
Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic dermatitis Is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139:1480–9.
Aridogan BC, Yildirim M, Baysal V, Inaloz HS, Baz K, Kaya S. Serum levels of IL-4, IL-10, IL-12, IL-13 and IFN-gamma in Behçet’s disease. J Dermatol. 2003;30:602–7.
Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–32.
Hasegawa M, Sato S, Nagaoka T, Fujimoto M, Takehara K. Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology. 2003;207:141–7.
Korenblat P, Kerwin E, Leshchenko I, Yen K, Holweg CTJ, Anzures-Cabrera J, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med. 2018;134:143–9.
Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411–20.
Panettieri RA, Sjöbring U, Péterffy A, Wessman P, Bowen K, Piper E, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med. 2018;6:511–25.
Shen L, Suresh L, Li H, Zhang C, Kumar V, Pankewycz O, et al. IL-14 alpha, the nexus for primary Sjögren’s disease in mice and humans. Clin Immunol. 2009;130:304–12.
Kiratiseavee S, Shen L, Baer AN, Ambrus JL. IL-14 RNA levels in the peripheral blood leukocytes of patients with SLE. J Allergy Clin Immunol. 2006;117:S18.
Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32.
Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
Willerslev-Olsen A, Litvinov IV, Fredholm SM, Petersen DL, Sibbesen NA, Gniadecki R, et al. IL-15 and IL-17F are differentially regulated and expressed in mycosis fungoides (MF). Cell Cycle. 2014;13:1306–12.
Chamie K, Chang S, Gonzalgo ML, Kramolowsky EV, Sexton WJ, Reddy SK, et al. Phase II/III clinical results of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients. JCO. 2021;39:510–510.
Asadullah K, Haeussler-Quade A, Gellrich S, Hanneken S, Hansen-Hagge TE, Döcke WD, et al. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: stage-dependent increase in mycosis fungoides progression. Exp Dermatol. 2000;9:248–51.
Kawabata K, Makino T, Makino K, Kajihara I, Fukushima S, Ihn H. IL-16 expression is increased in the skin and sera of patients with systemic sclerosis. Rheumatology (Oxford). 2020;59:519–23.
Frezzolini A, Cianchini G, Ruffelli M, Cadoni S, Puddu P, De Pità O. Interleukin-16 expression and release in bullous pemphigoid. Clin Exp Immunol. 2004;137:595–600.
Lard LR, Roep BO, Verburgh CA, Zwinderman AH, Huizinga TWJ. Elevated IL-16 levels in patients with systemic lupus erythematosus are associated with disease severity but not with genetic susceptibility to lupus. Lupus. 2002;11:181–5.
Amiel C, Darcissac E, Truong MJ, Dewulf J, Loyens M, Mouton Y, et al. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179:83–91.
Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–80.
Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–8.
Hohenberger M, Cardwell LA, Oussedik E, Feldman SR. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat. 2018;29:13–8.
Zhao J, Chen X, Herjan T, Li X. The role of interleukin-17 in tumor development and progression. J Exp Med. 2020;217: e20190297.
Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–5.
Wong CK, Li EK, Ho CY, Lam CW. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology (Oxford). 2000;39:1078–81.
Mazodier K, Marin V, Novick D, Farnarier C, Robitail S, Schleinitz N, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–9.
Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Foley E, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–35.
Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–7.
Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen-Solal A, et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004;18:1752–4.
Oka T, Sugaya M, Takahashi N, Nakajima R, Otobe S, Kabasawa M, et al. Increased interleukin-19 expression in cutaneous T-cell lymphoma and atopic dermatitis. Acta Derm Venereol. 2017;97:1172–7.
Wong CK, Wong PTY, Tam LS, Li EK, Chen DP, Lam CWK. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30:45–52.
Festen EM, Goyette P, Scott R, Annese V, Zhernakova A, Lian J, et al. Genetic variants in the region harbouring IL2/IL21 associated with ulcerative colitis. Gut. 2009;58:799–804.
Jin H, Oyoshi MK, Le Y, Bianchi T, Koduru S, Mathias CB, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J Clin Invest. 2009;119:47–60.
Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–7.
Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–22.
Costanzo A, Chimenti MS, Botti E, Caruso R, Sarra M, Monteleone G. IL-21 in the pathogenesis and treatment of skin diseases. J Dermatol Sci. 2010;60:61–6.
Stolfi C, Rizzo A, Franzè E, Rotondi A, Fantini MC, Sarra M, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208:2279–90.
Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol. 2010;71:334–41.
Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQF, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54.
Ricciardi L, Minciullo PL, Saitta S, Trombetta D, Saija A, Gangemi S. Increased serum levels of IL-22 in patients with nickel contact dermatitis. Contact Dermat. 2009;60:57–8.
Larsen JM, Bonefeld CM, Poulsen SS, Geisler C, Skov L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J Allergy Clin Immunol. 2009;123:486–92.
Mathian A, Parizot C, Dorgham K, Trad S, Arnaud L, Larsen M, et al. Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis. 2012;71:1227–34.
Miyagaki T, Sugaya M, Suga H, Kamata M, Ohmatsu H, Fujita H, et al. IL-22, but not IL-17, dominant environment in cutaneous T-cell lymphoma. Clin Cancer Res. 2011;17:7529–38.
Zhang S, Fujita H, Mitsui H, Yanofsky VR, Fuentes-Duculan J, Pettersen JS, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS ONE. 2013;8: e62154.
Perriard G, Mathias A, Enz L, Canales M, Schluep M, Gentner M, et al. Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes. J Neuroinflamm. 2015;12:1–18.
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–46.
Geremia A, Arancibia-Cárcamo CV, Fleming MPP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–33.
Brentano F, Ospelt C, Stanczyk J, Gay RE, Gay S, Kyburz D. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann Rheum Dis. 2009;68:143–50.
Gottlieb A, Menter A, Mendelsohn A, Shen Y-K, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–40.
Van Belle AB, Cochez PM, de Heusch M, Pointner L, Opsomer R, Raynaud P, et al. IL-24 contributes to skin inflammation in para-phenylenediamine-induced contact hypersensitivity. Sci Rep. 2019;9:1–11.
Mitamura Y, Nunomura S, Nanri Y, Ogawa M, Yoshihara T, Masuoka M, et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73:1881–91.
Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. 2009;183:687–95.
Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6: 256ra134.
Tang W, Smith SG, Salter B, Oliveria JP, Mitchell P, Nusca GM, et al. Allergen-induced increases in interleukin-25 and interleukin-25 receptor expression in mature eosinophils from atopic asthmatics. Int Arch Allergy Immunol. 2016;170:234–42.
Scala E, Di Caprio R, Cacciapuoti S, Caiazzo G, Fusco A, Tortorella E, et al. A new T helper 17 cytokine in hidradenitis suppurativa: antimicrobial and proinflammatory role of interleukin-26. Br J Dermatol. 2019;181:1038–45.
Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10: e1001395.
Fujii M, Nishida A, Imaeda H, Ohno M, Nishino K, Sakai S, et al. Expression of Interleukin-26 is upregulated in inflammatory bowel disease. World J Gastroenterol. 2017;23:5519–29.
Dambacher J, Beigel F, Zitzmann K, De Toni EN, Göke B, Diepolder HM, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207–17.
Konradsen JR, Nordlund B, Levänen B, Hedlin G, Linden A. The cytokine interleukin-26 as a biomarker in pediatric asthma. Respir Res. 2016;17:1–4.
Kaabachi W, Bouali E, Berraïes A, Dhifallh IB, Hamdi B, Hamzaoui K, et al. Interleukin-26 is overexpressed in Behçet’s disease and enhances Th17-related -cytokines. Immunol Lett. 2017;190:177–84.
Savchenko L, Mykytiuk M, Cinato M, Tronchere H, Kunduzova O, Kaidashev I. IL-26 in the induced sputum is associated with the level of systemic inflammation, lung functions and body weight in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2569–75.
Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Muroi E, et al. Elevated serum interleukin-27 levels in patients with systemic sclerosis: association with T cell, B cell and fibroblast activation. Ann Rheum Dis. 2011;70:194–200.
Cao J, Zhang L, Li D, Xu F, Huang S, Xiang Y, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest. 2012;141:121–30.
Lu D, Zhou X, Yao L, Liu C, Jin F, Wu Y. Clinical implications of the interleukin 27 serum level in breast cancer. J Investig Med. 2014;62:627–31.
Xia L, Shen H, Zhao L, Lu J. Elevated levels of interleukin-27 in patients with Sjögren’s syndrome. Scand J Rheumatol. 2012;41:73–4.
Tanida S, Yoshitomi H, Ishikawa M, Kasahara T, Murata K, Shibuya H, et al. IL-27-producing CD14(+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine. 2011;55:237–44.
Luo Q, Liu Y, Liu S, Yin Y, Xu B, Cao J. Interleukin 28 is a potential therapeutic target for sepsis. Clin Immunol. 2019;205:29–34.
Lin S-C, Kuo C-C, Tsao J-T, Lin L-J. Profiling the expression of interleukin (IL)-28 and IL-28 receptor α in systemic lupus erythematosus patients. Eur J Clin Invest. 2012;42:61–9.
Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:1–18.
Arpaci D, Karakas Celik S, Can M, Cakmak Genc G, Kuzu F, Unal M, et al. Increased serum levels of IL-28 and IL-29 and the protective effect of IL28B rs8099917 polymorphism in patients with Hashimoto’s thyroiditis. Immunol Invest. 2016;45:668–78.
Wu Q, Yang Q, Lourenco E, Sun H, Zhang Y. Interferon-lambda1 induces peripheral blood mononuclear cell-derived chemokines secretion in patients with systemic lupus erythematosus: its correlation with disease activity. Arthritis Res Ther. 2011;13:R88.
Fedenko ES, Elisyutina OG, Filimonova TM, Boldyreva MN, Burmenskaya OV, Rebrova OY, et al. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self Nonself. 2011;2:120–4.
Dantas AT, Gonçalves SMC, Pereira MC, de Almeida AR, Marques CDL, Rego MJBM, et al. Interferons and systemic sclerosis: correlation between interferon gamma and interferon-lambda 1 (IL-29). Autoimmunity. 2015;48:429–33.
Wang F, Xu L, Feng X, Guo D, Tan W, Zhang M. Interleukin-29 modulates proinflammatory cytokine production in synovial inflammation of rheumatoid arthritis. Arthritis Res Ther. 2012;14:R228.
Xu L, Peng Q, Xuan W, Feng X, Kong X, Zhang M, et al. Interleukin-29 enhances synovial inflammation and cartilage degradation in osteoarthritis. Mediat Inflamm. 2016;2016:9631510.
Haasnoot A-MJW, Kuiper JJW, Hiddingh S, Schellekens PAWJF, de Jager W, Imhof SM, et al. Ocular fluid analysis in children reveals interleukin-29/interferon-λ1 as a biomarker for juvenile idiopathic arthritis-associated uveitis. Arthritis Rheumatol. 2016;68:1769–79.
Apostolou E, Kapsogeorgou EK, Konsta OD, Giotakis I, Saridaki MI, Andreakos E, et al. Expression of type III interferons (IFNλs) and their receptor in Sjögren’s syndrome. Clin Exp Immunol. 2016;186:304–12.
Airoldi I, Cocco C, Sorrentino C, Angelucci D, Di Meo S, Manzoli L, et al. Interleukin-30 promotes breast cancer growth and progression. Cancer Res. 2016;76:6218–29.
Di Meo S, Airoldi I, Sorrentino C, Zorzoli A, Esposito S, Di Carlo E. Interleukin-30 expression in prostate cancer and its draining lymph nodes correlates with advanced grade and stage. Clin Cancer Res. 2014;20:585–94.
Bağci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858–66.
Yao Q, Wang B, Jia X, Li Q, Yao W, Zhang J-A. Increased human interleukin-32 expression is related to disease activity of Graves’ disease. Front Endocrinol (Lausanne). 2019;10:1–7.
Di Benedetto P, Guggino G, Manzi G, Ruscitti P, Berardicurti O, Panzera N, et al. Interleukin-32 in systemic sclerosis, a potential new biomarker for pulmonary arterial hypertension. Arthritis Res Ther. 2020;22:1–9.
Meyer N, Zimmermann M, Bürgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:858-865.e10.
Joosten LAB, Netea MG, Kim S-H, Yoon D-Y, Oppers-Walgreen B, Radstake TRD, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–303.
Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901.
Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–400.
Mattii M, Ayala F, Balato N, Filotico R, Lembo S, Schiattarella M, et al. The balance between pro- and anti-inflammatory cytokines is crucial in human allergic contact dermatitis pathogenesis: the role of IL-1 family members. Exp Dermatol. 2013;22:813–9.
Kay AB, Clark P, Maurer M, Ying S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous ('idiopathic’) urticaria. Br J Dermatol. 2015;172:1294–302.
Li P, Ma H, Han D, Mou K. Interleukin-33 affects cytokine production by keratinocytes in vitiligo. Clin Exp Dermatol. 2015;40:163–70.
Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–81.
Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103.
Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, Nagatani K, et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J Rheumatol. 2010;37:18–25.
Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007.
Moon S-J, Hong Y-S, Ju JH, Kwok S-K, Park S-H, Min J-K. Increased levels of interleukin 34 in serum and synovial fluid are associated with rheumatoid factor and anticyclic citrullinated peptide antibody titers in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1842–9.
Xie HH, Shen H, Zhang L, Cui MY, Xia LP, Lu J. Elevated serum interleukin-34 level in patients with systemic lupus erythematosus is associated with disease activity. Sci Rep. 2018;8:1–6.
Ciccia F, Alessandro R, Rodolico V, Guggino G, Raimondo S, Guarnotta C, et al. IL-34 is overexpressed in the inflamed salivary glands of patients with Sjögren’s syndrome and is associated with the local expansion of pro-inflammatory CD14(bright)CD16+ monocytes. Rheumatology (Oxford). 2013;52:1009–17.
Franzè E, Monteleone I, Cupi ML, Mancia P, Caprioli F, Marafini I, et al. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci (Lond). 2015;129:271–80.
Shoji H, Yoshio S, Mano Y, Kumagai E, Sugiyama M, Korenaga M, et al. Interleukin-34 as a fibroblast-derived marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6:28814.
Baghdadi M, Endo H, Takano A, Ishikawa K, Kameda Y, Wada H, et al. High co-expression of IL-34 and M-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep. 2018;8:1–10.
Fogel O, Rivière E, Seror R, Nocturne G, Boudaoud S, Ly B, et al. Role of the IL-12/IL-35 balance in patients with Sjögren syndrome. J Allergy Clin Immunol. 2018;142:258-268.e5.
Jafarzadeh A, Jamali M, Mahdavi R, Ebrahimi HA, Hajghani H, Khosravimashizi A, et al. Circulating levels of interleukin-35 in patients with multiple sclerosis: evaluation of the influences of FOXP3 gene polymorphism and treatment program. J Mol Neurosci. 2015;55:891–7.
Tomcik M, Zerr P, Palumbo-Zerr K, Storkanova H, Hulejova H, Spiritovic M, et al. Interleukin-35 is upregulated in systemic sclerosis and its serum levels are associated with early disease. Rheumatology (Oxford). 2015;54:2273–82.
Chu M, Wong CK, Cai Z, Dong J, Jiao D, Kam NW, et al. Elevated expression and pro-inflammatory activity of il-36 in patients with systemic lupus erythematosus. Molecules. 2015;20:19588–604.
Frey S, Derer A, Messbacher M-E, Baeten DLP, Bugatti S, Montecucco C, et al. The novel cytokine interleukin-36α is expressed in psoriatic and rheumatoid arthritis synovium. Ann Rheum Dis. 2013;72:1569–74.
Russell SE, Horan RM, Stefanska AM, Carey A, Leon G, Aguilera M, et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016;9:1193–204.
Ciccia F, Accardo-Palumbo A, Alessandro R, Alessandri C, Priori R, Guggino G, et al. Interleukin-36α axis is modulated in patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2015;181:230–8.
Suárez-Fariñas M, Ungar B, da Rosa JC, Ewald DA, Rozenblit M, Gonzalez J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol. 2015;135:1218–27.
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med. 2014;12:1–8.
Chen B, Huang K, Ye L, Li Y, Zhang J, Zhang J, et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J Transl Med. 2015;13:1–9.
Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang K, et al. Increased expression of IL-37 in patients with Graves’ disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PLoS ONE. 2014;9: e107183.
Li C, Zhao P, Sun X, Che Y, Jiang Y. Elevated levels of cerebrospinal fluid and plasma interleukin-37 in patients with Guillain-Barré syndrome. Mediat Inflamm. 2013;2013: 639712.
Zhao P-W, Jiang W-G, Wang L, Jiang Z-Y, Shan Y-X, Jiang Y-F. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS ONE. 2014;9: e95346.
Boutet M-A, Bart G, Penhoat M, Amiaud J, Brulin B, Charrier C, et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184:159–73.
Tominaga M, Okamoto M, Kawayama T, Matsuoka M, Kaieda S, Sakazaki Y, et al. Overexpression of IL-38 protein in anticancer drug-induced lung injury and acute exacerbation of idiopathic pulmonary fibrosis. Respir Investig. 2017;55:293–9.
Yang M-G, Tian S, Zhang Q, Han J, Liu C, Zhou Y, et al. Elevated serum interleukin-39 levels in patients with neuromyelitis optica spectrum disorders correlated with disease severity. Mult Scler Relat Disord. 2020;46: 102430.
Luo Y, Liu F, Liu H, Chen H, Cheng W, Dong S, et al. Elevated serum IL-39 in patients with ST-segment elevation myocardial infarction was related with left ventricular systolic dysfunction. Biomark Med. 2017;11:419–26.
Acknowledgements
Funding
Publication fees were covered by Bausch Health Canada Inc.
Medical Writing and Editorial Assistance
The authors wish to acknowledge Houssam Ismail, PhD, for providing medical writing assistance with this manuscript, which was funded by Bausch Health Canada, Inc. Dr. Ismail is a freelance medical writer employed by STA HealthCare Communications. The authors also would like to thank Dr. Yuka Asai for critical reading of the manuscript.
Author Contributions
I.T. and M.B. conceived and designed the outline of the work, and co-wrote the manuscript.
Disclosures
Dr. Turchin was a consultant, speaker and investigator for AbbVie, Amgen, Arcutis, Aristea, Bausch Health, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Incyte, Janssen, Kiniksa, LeoPharma, Novartis, Pfizer, Sanofi, UCB. Dr. Bourcier was a consultant speaker for AbbVie, Amgen-Wyeth, Bausch Health, Celgene, Eli-Lilly, GSK, Janssen, LEO Pharma, Pfizer Novartis, Sanofi, UCB and was an advisor for AbbVie, Amgen-Wyeth, Bausch Health, BMS, Celgene, Eli-Lilly, Janssen, LEO Pharma, Pfizer, Novartis, Sanofi, UCB. He has been a principal investigator for the following companies: AbbVie, Amgen, Celgene, Eli-Lilly, Janssen, Leo, Novartis.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
There are no data associated with this review article apart from what is presented and referenced here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turchin, I., Bourcier, M. The Role of Interleukins in the Pathogenesis of Dermatological Immune-Mediated Diseases. Adv Ther 39, 4474–4508 (2022). https://doi.org/10.1007/s12325-022-02241-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02241-y