Abstract
Introduction
The VISIONARY study demonstrated statistically significant intraocular pressure (IOP) reductions with the preservative-free fixed-dose combination of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC) in open-angle glaucoma (OAG) or ocular hypertension (OHT) patients, sub-optimally controlled with topical prostaglandin analogue (PGA) or beta-blocker monotherapy. Current subanalyses have examined these data according to the baseline monotherapy.
Methods
A European, prospective, observational study included adults (aged ≥ 18 years) with OAG or OHT, who were switched to the PF tafluprost/timolol FC from PGA or beta-blocker monotherapy. Treatment outcomes were reported according to prior monotherapy subgroup: beta-blocker, preserved latanoprost, PF-latanoprost, bimatoprost, tafluprost, and travoprost. Endpoints included the mean change from baseline regarding IOP, conjunctival hyperemia, and corneal fluorescein staining (CFS) at Week 4 and Week 12, and at Month 6.
Results
The subanalysis included 577 patients. All prior monotherapy subgroups demonstrated statistically significant IOP reductions from baseline at Week 4, that were maintained through Month 6 (p < 0.001). Mean (SD) IOP change at Month 6 was 6.6 (4.16), 6.3 (4.39), 5.6 (3.67), 4.9 (2.97), 4.6 (4.39), and 4.7 (3.64) mmHg for prior beta-blocker, preserved latanoprost, PF-latanoprost, tafluprost, bimatoprost, and travoprost subgroups, respectively. The largest IOP change was observed in the preserved latanoprost subgroup for each of the ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35% IOP reduction categories at Month 6, demonstrating respective reductions of 8.06, 9.20, 10.64, and 11.55 mmHg. CFS was significantly reduced at Month 6 in the prior bimatoprost subgroup (p = 0.0013). Conjunctival hyperemia severity was significantly reduced at each study visit for prior preserved latanoprost users (p < 0.001).
Conclusion
PF tafluprost/timolol FC therapy provided statistically and clinically significant IOP reductions from Week 4 over the total 6-month period, in patients with OAG/OHT, regardless of the type of prior PGA or beta-blocker monotherapy used. Conjunctival hyperemia severity and CFS decreased significantly in prior bimatoprost and preserved latanoprost users, respectively.
Clinical Study Number
European Union electronic Register of Post-Authorization Studies (EU PAS) register number: EUPAS22204.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Reduction of intraocular pressure (IOP) is the only modifiable risk factor for disease progression and irreversible sight loss in open-angle glaucoma (OAG). |
The preservative-free fixed-dose combination of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC) has demonstrated IOP-lowering efficacy alongside good tolerability in randomized controlled trials and real-world studies conducted in OAG or ocular hypertension (OHT) patients, including the most recent observational data from the VISIONARY study. |
The current subanalysis of data from the VISIONARY study examines the influence of prior beta-blocker monotherapy and each prostaglandin analogue (PGA) monotherapy molecule and formulation on treatment outcomes in OAG/OHT following a switch to PF tafluprost/timolol FC therapy. |
What was learned from the study? |
Subanalysis of data from the visionary study according to the monotherapy used at baseline showed that a treatment switch to the PF tafluprost/timolol FC was associated with statistically and clinically significant IOP reductions, that were evident from Week 4 and maintained over the 6-month study period for patients in all prior monotherapy subgroups (various PGA molecules and a beta-blocker) (p < 0.001). |
Mean change in IOP from baseline at Month 6 ranged between 4.6 mmHg (20.5%) in the prior bimatoprost subgroup and 6.3 mmHg (26.3%) in the preserved latanoprost subgroup. Patients in the prior bimatoprost subgroup demonstrated significant reductions in corneal fluorescein staining (p = 0.0013) at Month 6 after initiating PF tafluprost/timolol FC therapy, and conjunctival hyperemia severity was significantly reduced in the preserved latanoprost subgroup (p < 0.001). |
The study provides insights regarding the treatment outcomes that may be expected when switching from a beta-blocker or a PGA monotherapy to the PF tafluprost/timolol FC. All prior monotherapy subgroups achieved IOP reductions of at least 20% from baseline, with the greatest change in IOP seen in the preserved latanoprost group which also had the highest baseline IOP. |
Introduction
Treatment of ocular hypertension (OHT) and open-angle glaucoma (OAG) focuses on powerful reduction of elevated intraocular pressure (IOP), which is the most important modifiable risk factor for glaucoma progression and glaucoma-related irreversible loss of vision [1,2,3,4,5,6]. Guidelines recommend that topical IOP-lowering monotherapies be used as first-line treatment [3, 5]. Fixed-dose combination (FC) formulations, typically comprising a prostaglandin analogue (PGA) and a beta-receptor blocker (beta-blocker), are recommended for treatment intensification when more active ingredients are necessary to reach the target IOP [3, 7, 8]. FC formulations both simplify the treatment regimen and reduce exposure of the ocular surface to toxic preservatives [3, 5]. In particular, FC formulations help to lower exposure to the most commonly used preservative found in topical glaucoma therapy—benzalkonium chloride (BAK) [3, 5, 9,10,11,12,13]. Preservative-free (PF) FC topical drug formulations provide additional benefits to those of the preserved FCs, since they completely prevent preservative-related ocular surface alterations, and may therefore improve tolerance and support enhanced treatment adherence [5, 12,13,14,15,16].
The PF FC formulation of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC; Santen OY, Finland) has been widely used in the treatment of OAG or OHT since 2014. The clinical value of PF tafluprost/timolol FC has been shown in both randomized controlled trials and observational studies [13, 15,16,17,18,19,20,21,22,23]. Most recently, the VISIONARY study, a large European prospective observational study, demonstrated statistically and clinically significant IOP reduction with PF tafluprost/timolol FC treatment in OAG or OHT patients, who were insufficiently controlled with or unable to tolerate a topical PGA or beta-blocker monotherapy [23]. The key signs and symptoms of ocular surface health were also improved following the initiation of PF tafluprost/timolol FC treatment [23]. The VISIONARY study examined prospectively recorded clinical data from 577 participants in 11 European countries (66 sites) over a 6-month period [23]. The published results presented data for the overall study population, and demonstrated clinically meaningful and statistically significant improvements in IOP and the signs of ocular health, regardless of the type of the first-line monotherapy (PGA or beta-blocker) used at baseline [23]. These real-world data provide an indication of the IOP reduction that clinicians may see in their own clinical practice when switching medication from a PGA or beta-blocker monotherapy to PF tafluprost/timolol FC therapy [23]. Real-world studies have become increasingly recognized as an important tool in understanding how patients may tolerate direct treatment switches in clinical practice, providing important pharmacovigilance data and expanding the evidence base to inform treatment pathways [24,25,26].
The majority (72.1%) of patients included in the VISIONARY study population were treated with a PGA monotherapy prior to initiating PF tafluprost/timolol FC therapy. Therefore, in the current analysis, we separately present the IOP reduction results (absolute and percentage IOP reduction, and responder rates) according to the type of PGA monotherapy used before initiating the PF tafluprost/timolol FC. In addition, most PGA users in the VISIONARY study had been prescribed latanoprost prior to baseline, and the high patient numbers in this group enabled further subanalysis to be conducted, in which treatment outcomes were assessed separately for those receiving preserved latanoprost and PF-latanoprost formulations, to explore the potential impact of prior chronic exposure to BAK. Therefore, the change in conjunctival hyperemia severity and corneal fluorescein staining (CFS) during the study period are also presented according to each monotherapy used at baseline. Finally, ophthalmologist-evaluated observations of IOP-lowering effectiveness, clinical signs and treatment compliance, and patient-assessed tolerability are reported for each of the pre-study monotherapy subgroups. This subanalysis aims to provide further insights and clarity regarding the treatment outcomes that may be expected when stepping up to the PF tafluprost/timolol FC from prior PGA or beta-blocker therapy in routine clinical practice.
Methods
Study Design and Visit Schedule
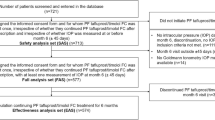
The design and methods of the VISIONARY study have previously been described in detail [23]. Briefly, this was a 6-month, observational, multicenter, European, prospective clinical study. The institutional review board (IRB) or independent ethics committee (IEC) at each center approved the study protocol, which was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) European Union electronic Register of Post-Authorisation Studies (EU PAS, Register number EUPAS22204). The study complied with the principles of the Declaration of Helsinki and all patients provided written informed consent before enrollment.
Prospective data collection was conducted during routine ophthalmology appointments at 66 participating ophthalmology clinics in Denmark, Hungary, Ireland, Italy, Latvia, Netherlands, Norway, Russia, Spain, Sweden, and the United Kingdom (between April 2017 and January 2019). Attendance at baseline and Month 6 visits was compulsory for study participation, although data were also recorded at interim visits (Week 4 and Week 12) where patients chose to attend. Baseline measures were recorded under a topical PGA or a beta-blocker monotherapy within 7 days of implementing the switch to PF tafluprost/timolol FC treatment.
Patient Population and Study Treatment
Male/female adults (aged ≥ 18 years) with a diagnosis of OAG or OHT were included in the study. In accordance with the licensed indication for PF tafluprost/timolol FC, participants had demonstrated either insufficient IOP control or poor tolerance with their current PGA or beta-blocker monotherapy, and were considered likely to benefit from the use of a PF topical formulation according to the judgement of the investigator ophthalmologist [23, 27].
Participants had not undergone ophthalmic surgery within 6 months prior to the study period, and had never received previous PF tafluprost/timolol FC treatment. Patients who were pregnant or breast-feeding at the screening visit, and those with any contraindication against tafluprost or timolol treatment according to the approved licensed indication, were not included in the study.
During the 6-month study period, the participants treated their affected eye(s) with the PF tafluprost/timolol FC (one drop daily). Study treatment was instilled either in the morning or in the evening, based on patient preference.
Assessment of Exploratory Endpoints
Endpoints examined in the current analysis were based upon data reported for the VISIONARY study full analysis set (FAS) population, and examined treatment outcomes according to the monotherapy (PGA or beta-blocker) used before initiating PF tafluprost/timolol FC treatment [23]. Where available, data were examined for each of the following prior monotherapy subgroups: all PGA therapies, beta-blocker therapies, preserved latanoprost, PF-latanoprost, bimatoprost, tafluprost, and travoprost. Based on the available formulation in each of the countries that participated in the study, preserved and PF PGA formulations were used at baseline. Subject numbers were sufficient to allow a subdivision of the latanoprost subgroup to PF and preserved formulations of this PGA monotherapy, but numbers were not high enough to enable further analysis in the subgroups for other PGA therapies.
The reasons for initiating PF tafluprost/timolol FC treatment were analyzed according to the type of prior monotherapy used. The protocol allowed the investigator to indicate more than one reason for patient selection. The categories comprised insufficient IOP control, progression of glaucoma on the current monotherapy, conversion of OHT to OAG, poor local tolerance of the current topical medication, insufficient adherence to the medication used, or “other reasons”.
The mean (standard deviation [SD]) IOP change from baseline was measured at Week 4, Week 12, and Month 6 using Goldmann applanation tonometry. The mean absolute (mmHg) and relative (%) IOP change from baseline was reported at Week 4, Week 12, and Month 6 according to prior monotherapy. Responders were defined as patients achieving an IOP reduction of at least 20% from baseline at Week 12, and further analysis was conducted to explore Month 6 IOP reductions of ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35% from baseline. The Month 6 mean absolute (mmHg) and relative (%) IOP change from baseline was reported for all patients included in the FAS, as well as for patients in each of the following prior monotherapy subgroups: beta-blockers, preserved latanoprost, PF-latanoprost, bimatoprost, tafluprost, and travoprost.
Evaluation of CFS (Oxford Grade Scale; grades 0–V) was conducted at all study visits [28]. The change in CFS score from baseline was reported for all prior PGA and beta-blocker users, as well as for patients previously treated with preserved latanoprost, PF-latanoprost, bimatoprost, tafluprost, and travoprost. The change from baseline in conjunctival hyperemia severity (graded as none, mild, moderate, or severe) was evaluated for each study visit, and was compared between patients treated with preserved latanoprost and PF-latanoprost.
Investigator and Patient Assessments
Investigator evaluation and patient-assessed data were analyzed according to the prior monotherapy subgroup. Investigators reported their evaluation of IOP-lowering effectiveness, clinical signs, and treatment compliance with PF tafluprost/timolol FC at Month 6, compared with baseline monotherapy, using a 3-point scale (better than prior therapy, same as prior therapy, worse than prior therapy). Patient-assessed tolerability with PF tafluprost/timolol FC therapy at Month 6 was reported using a 4-point scale (very good, good, satisfactory, poor).
Statistical Analysis
ICON (Dublin, Ireland) conducted all statistical analyses on behalf of the VISIONARY study group [23]. Data distribution was assessed using Q–Q plots, histograms, and the Shapiro–Wilk or the Kolmogorov–Smirnov tests, as needed. For normally distributed data, the mean and SD values were reported, and the paired t test was used for the comparisons. A linear mixed model was used with IOP as the dependent variable and all timepoints as independent variables to compare the IOP change between each study visit and the baseline value, separately, using the paired t test. Change from baseline concerning CFS and conjunctival hyperemia was assessed using the Bhapkar test [29]. The p value used as the cut-off for statistical significance was < 0.05.
Results
Study Population Demographics and Reasons for a Change in Treatment
As reported previously by Oddone et al. [23], 721 participants were screened for inclusion in the study and 713 were treated with the PF tafluprost/timolol FC. Of these, 577 went on to complete the 6-month visit and were included in the analysis [23].
Baseline demographics are shown in Table 1. The analysis included 577 patients (FAS). Mean (SD) age was 67.8 (11.67; range 23.7–96.1) years, and 59.6% of the final population were female. At baseline most participants (72.1%) were treated with a PGA and 27.9% with beta-blocker monotherapy. Of those treated with a PGA monotherapy at baseline, preserved latanoprost was the most frequently used (27.6%), while 15.1%, 11.1%, 10.9% and 7.3% of the participants used tafluprost, bimatoprost, travoprost and PF-latanoprost, respectively.
Table 2 shows the reasons reported for changing the prior monotherapy to PF tafluprost/timolol FC therapy. Insufficient IOP control was the most common reason for switching therapy in all prior monotherapy subgroups: PF-latanoprost (95.2%), preserved latanoprost (81.8%), tafluprost (85.1%), bimatoprost (70.3%), travoprost (66.7%), and beta-blockers (85.1%). Poor local tolerance was the second most common reason for the treatment switch in prior users of bimatoprost (35.9%), travoprost (30.2%), and preserved latanoprost (23.9%). Progression of glaucoma was reported as the second most popular reason for switch in the beta-blocker (16.1%) and PF-latanoprost (4.8%) subgroups, while this was the third most common reason given for patients in the travoprost (20.6%), preserved latanoprost (17.0%), tafluprost (16.1%), and bimatoprost (12.5%) subgroups.
Subanalysis of Intraocular Pressure Data
Data concerning the change in mean (SD) IOP from baseline according to the type of the prior monotherapy (PGA or beta-blocker) are shown in Table 3. Statistically significant IOP reductions were observed at Week 4 that were sustained through the 6-month study period, regardless of the prior monotherapy subgroup (p < 0.001). When examining the mean (SD) IOP change from baseline at Month 6 for all PGA and beta-blocker users, respective reductions of 5.4 (4.04) mmHg and 6.6 (4.16) mmHg were observed (p < 0.0001 for both subgroups). Mean (SD) IOP reductions from baseline in each prior PGA monotherapy subgroup at Month 6 were 6.3 (4.39) mmHg (p < 0.001; preserved latanoprost), 5.6 mmHg (3.67) mmHg (p < 0.001; PF-latanoprost), 4.9 mmHg (2.97) mmHg (p < 0.0001; tafluprost), 4.6 mmHg (4.39) mmHg (p < 0.0001; bimatoprost), and 4.7 mmHg (3.64) mmHg (p < 0.0001; travoprost). Mean (SD) reduction from baseline at Month 6 for users of all latanoprost formulations (preserved and PF) was 6.1 (4.25) mmHg (25.9%; p < 0.0001). The change in mean IOP at Month 6 was 26.3%, 24.6%, 22.7%, 20.5%, and 21.3% for prior preserved latanoprost, PF-latanoprost, tafluprost, bimatoprost, and travoprost users, respectively.
Table 4 presents the mean IOP change from baseline and the responder rates at Month 6 for patients achieving an IOP reduction of ≥ 20%, ≥ 25%, ≥ 30%, or ≥ 35% from baseline. Data are shown for all study participants, all beta-blocker users, and for each of the prior PGA therapy subgroups. Overall, 77.6%, 62.7%, 49.1%, and 36.0% of patients using beta-blocker monotherapy at baseline achieved an IOP reduction of ≥ 20% ≥ 25%, ≥ 30%, and ≥ 35%, respectively, at Month 6. The mean IOP on prior monotherapy was highest for patients treated with preserved latanoprost at baseline (22.4 mmHg), and the absolute IOP reduction in this group was the greatest of the prior PGA subgroups. Mean IOP was reduced by 8.06, 9.20, 10.64, and 11.55 mmHg at Month 6 for responders achieving IOP reductions of ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35%, respectively. The percentage of responders in each of the prior PGA subgroups achieving an IOP reduction ≥ 20% was 69.8% (preserved latanoprost), 66.7% (PF-latanoprost), 64.4% (tafluprost), 63.5% (travoprost), and 60.9% (bimatoprost). An IOP reduction ≥ 25% was achieved at Month 6 by 54.8%, 52.2%, 50.0%, 47.6%, and 46.0% in the prior PF-latanoprost, preserved latanoprost, bimatoprost, travoprost, and tafluprost subgroups, respectively. The percentage of responders in each PGA subgroup with an IOP reduction ≥ 30% at Month 6 was 42.9% (PF-latanoprost), 36.5% (preserved latanoprost), 34.9% (travoprost), 34.4% (bimatoprost), and 29.9% (tafluprost). The percentage of responders with an IOP reduction ≥ 35% in each of the PGA subgroups was 27.7% (preserved latanoprost), 23.4% (bimatoprost), 21.4% (PF-latanoprost), 16.1% (tafluprost), and 14.3% (travoprost).
Change in Corneal Fluorescein Staining (CFS) According to Baseline Monotherapy
Table 5 shows the mean (SD) change in CFS score at each study visit according to the prior monotherapy subgroup. The mean baseline CFS score was typically < 1.0 in each of the subgroups examined with the exception of the prior bimatoprost group, in which the mean (SD) CFS score was 1.34 (1.16) before initiating PF tafluprost/timolol FC therapy.
Overall, mean (SD) CFS score was reduced from 0.82 (0.97) at baseline to 0.49 (0.73; p = 0.0001) at Month 6 for all prior PGA users and from 0.48 (0.75) at baseline to 0.35 (0.59; p = 0.1907) at Month 6 for prior beta-blocker users. Numerical reductions in CFS score were observed at Month 6 in each of the prior PGA monotherapy subgroups. However, the reduction in CFS score was only statistically significant in the prior bimatoprost subgroup, in which a mean (SD) reduction from baseline at Month 6 of 0.71 (1.17) was observed (p = 0.0013).
Change in Conjunctival Hyperemia According to Baseline Monotherapy
Figure 1 shows the change in the severity of conjunctival hyperemia at each study visit for patients treated with all prior PGA monotherapies and beta-blockers at baseline, separately. Change in conjunctival hyperemia was statistically significant at each study visit among prior PGA users (p < 0.0001). Hyperemia was absent in 30.3% of PGA users at baseline and 53.8% at Month 6. Moderate hyperemia was reported in 21.3% of PGA users at baseline and 4.3% at Month 6. Conjunctival hyperemia was absent in 52.3% of prior beta-blockers at baseline and 61.7% at Month 6. The change in the severity of hyperemia was not statistically significant among baseline beta-blocker users (p = 0.5232).
Change in severity of conjunctival hyperemia following initiation of the preservative-free tafluprost/timolol fixed-dose combination according to the prior PGA or beta-blocker monotherapy use (full analysis set). a Change in severity of hyperemia from baseline following the initiation of PF tafluprost/timolol FC in prior PGA users: p < 0.0001 at each study visit. b Change in severity of hyperemia from baseline following the initiation of PF tafluprost/timolol FC in prior beta-blocker users: p = 0.6916 at Week 4, p = 0.5498 at Week 12, p = 0.5232 at Month 6. FC fixed combination, n number of patients, PF preservative-free, PGA prostaglandin analogue
Figure 2 shows the change in the severity of conjunctival hyperemia for prior preserved latanoprost and PF-latanoprost users. The change was statistically significant at each study visit for those in the preserved latanoprost subgroup (p < 0.001), whereas the change in conjunctival hyperemia severity was not significant during the study period for prior PF-latanoprost users. At baseline, 22.0% of prior preserved latanoprost users had moderate conjunctival hyperemia and this was reduced to 6.2% at Month 6, while the percentage with no hyperemia was increased from 28.0% at baseline to 46.9% at Month 6. Conjunctival hyperemia was typically mild or absent in prior PF-latanoprost users at both baseline (97.1%) and Month 6 (95%).
Change in severity of conjunctival hyperemia following a switch to the preservative-free tafluprost/timolol fixed-dose combination from preserved and preservative-free latanoprost monotherapy, respectively (full analysis set). a Change in severity of hyperemia from baseline following the initiation of PF tafluprost/timolol FC in prior preserved latanoprost users (n = 159): p < 0.001 at each visit. b Change in severity of hyperemia from baseline following the initiation of PF tafluprost/timolol FC in prior PF-latanoprost users (n = 42): p = 0.377 at Week 4, p = 0.472 at Week 12, p = 0.148 at Month 6. FC fixed combination, n number of patients, PF preservative-free
Investigator Evaluation of Clinical Effectiveness, Clinical Signs and Treatment Compliance
Figure 3a shows investigator evaluations at Month 6 regarding IOP-lowering effectiveness, clinical signs, and treatment compliance with PF tafluprost/timolol FC compared with baseline monotherapy for each of the PGA subgroups and for prior beta-blocker users. Investigators rated treatment effectiveness to be better with PF tafluprost/timolol FC for most patients (≥ 77%) in each of the prior treatment subgroups. Clinical signs with PF tafluprost/timolol FC treatment were considered to be either improved or the same as previous therapy in the majority of patients (> 95%) in each of the prior treatment subgroups. Treatment compliance was also reported to be improved or comparable with prior monotherapy in > 96% of patients in each of the prior treatment subgroups.
a Physician evaluation of effectiveness, clinical signs and treatment compliance after 6 months of treatment with preservative-free tafluprost/timolol fixed-dose combination therapy compared with prior prostaglandin analogue or beta-blocker monotherapy (full analysis set). b Patient evaluation of tolerability after 6 months of treatment with preservative-free tafluprost/timolol fixed-dose combination therapy (full analysis set). PF preservative-free
Figure 3b shows patient-assessed tolerability data for PF tafluprost/timolol FC treatment at Month 6, compared with prior monotherapy for each of the PGA subgroups and for prior beta-blocker users. More than 85% of patients in each subgroup evaluated tolerability with PF tafluprost/timolol FC to be good or very good (Fig. 3b).
Discussion
The current subanalysis of data from the VISIONARY study provides further evidence to support the IOP-lowering efficacy and tolerability of the PF tafluprost/timolol FC when used in a real-world setting. VISIONARY comprised a large, European, observational, multicenter study that examined treatment outcomes following a direct switch (without a washout period) to the PF tafluprost/timolol FC in patients with OAG or OHT, who were either insufficiently controlled with or unable to tolerate PGA or beta-blocker monotherapy [23]. The current subanalysis demonstrated that statistically significant IOP reductions were achieved from Week 4 through Month 6 in prior beta-blocker users, as well as in each of the prior PGA monotherapy subgroups examined. The study represents the largest European on-label real-world study to specifically examine the IOP lowering-efficacy of the PF tafluprost/timolol FC in patients switching directly from PGA or beta-blocker monotherapy, and the first to include subanalyses of these data according to the individual PGA treatment used at baseline. The high number of patients included in the study, who had been treated at baseline with preserved latanoprost, provides an indication of treatment practices in ophthalmology clinics across Europe, and the subanalyses reported here reveal the efficacy and tolerability outcomes that may be expected when switching to the PF tafluprost/timolol FC from each PGA monotherapy or from a topical beta-blocker.
Irrespective of the baseline monotherapy used (PGA or beta-blocker), most patients initiating PF tafluprost/timolol FC had been unable to achieve sufficient IOP control with monotherapy alone. Between 66% and 95% of participants in each baseline treatment subgroup were switched to PF tafluprost/timolol FC therapy because of poor IOP control. More than 23% of those previously treated with preserved latanoprost, bimatoprost, or travoprost were switched due to poor local tolerance, whereas tolerability issues were not cited as a reason for changing therapy among any prior PF-latanoprost users. These results are aligned with previous studies suggesting that issues regarding ocular surface tolerance may be due to either the presence of preservative agent or the active prostaglandin agent itself [21, 30]. It is interesting to note that patient-reported tolerability with PF tafluprost/timolol FC therapy was rated as good or very good by the majority of participants (> 85%) in each of the prior treatment subgroups, and this aspect of treatment is likely to have driven the high rates of compliance (as evaluated by investigators) reported in both the primary publication and the current subanalysis [23].
IOP subanalysis according to prior monotherapy showed that clinically meaningful and statistically significant IOP reductions from baseline were demonstrated at all study visits, regardless of the monotherapy used prior to initiating PF tafluprost/timolol FC treatment (p < 0.001). At Month 6, the mean IOP in each prior treatment subgroup was ≤ 16.5 mmHg, with the highest relative reductions from baseline seen in the prior preserved latanoprost subgroup (6.3 mmHg; 26.3%). Patients in all subgroups achieved a mean IOP reduction of at least 4.6 mmHg (20.5%). Regardless of the prior monotherapy subgroup, statistically and clinically significant IOP reductions were present from Week 4 and sustained over the 6-month study period. These data are reflective of the primary VISIONARY publication and of other real-world data concerning IOP-lowering efficacy with PF tafluprost/timolol FC following a switch away from different topical glaucoma medications [22, 23]. Emerging country-level data from the VISIONARY study has revealed differences in baseline IOP, with clinicians in some countries selecting patients with higher IOPs for inclusion in the study [31,32,33,34]. As pre-treatment pressure is predictive of IOP change, it is likely that these differences may have influenced the IOP reductions observed in the full Europe-wide dataset, and further subanalysis of these data according to country would be of value [16]. Improved adherence with PF tafluprost/timolol FC treatment, compared with prior monotherapy, may also have contributed to the IOP reductions and maintained IOP control observed during the current study. Clinicians rated compliance with PF tafluprost/timolol FC to be better than prior monotherapy in 44.2–64.5% patients previously treated with preserved latanoprost, bimatoprost, travoprost, or tafluprost. Approximately 30% of prior PF-latanoprost users were considered to have better compliance with treatment following the switch to PF tafluprost/timolol FC. The improvements seen concerning conjunctival hyperemia among those previously treated with preserved latanoprost, and the reduction in CFS score associated with a change to PF tafluprost/timolol FC from bimatoprost monotherapy, suggest that patients may have experienced tolerability issues with these PGA monotherapies, which might also have affected their adherence [21, 30, 35,36,37,38,39,40,41]. Patients who subsequently had better adherence with PF tafluprost/timolol FC therapy would therefore show improved IOP-lowering efficacy. There is a need for further data in this area to understand the impact of improved ocular tolerability on treatment efficacy with glaucoma medications. Phase 3 randomized studies examining PF tafluprost/timolol FC therapy also demonstrated statistically significant IOP-lowering efficacy, although those trials included a washout period prior to the treatment, which provided untreated baseline values and may have helped to minimize any tolerability issues associated with the prior therapies [15, 16, 19]. The current subanalysis offers an indication of the IOP reduction that clinicians may expect in their own clinics when directly switching therapy from either PGA or beta-blocker monotherapy. Subanalysis of IOP data according to the cut-off values of ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35% showed respective responder rates to be at least 60%, 46%, 29%, and 14%, across all prior monotherapy subgroups. For each of the preset IOP reduction categories, the greatest relative reductions were seen in the prior preserved latanoprost subgroup, where mean IOP reductions of 8.06, 9.20, 10.64, and 11.55 mmHg were reported for patients achieving IOP reductions of ≥ 20%, ≥ 25%, ≥ 30%, and ≥ 35%, respectively. Unfortunately, statistical analysis was not available to directly compare IOP-lowering outcomes between treatment groups, but this may represent an interesting area for further studies to inform future treatment pathways for OAG or OHT. The IOP-lowering efficacy demonstrated with PF tafluprost/timolol FC treatment during the study period was supported by the clinical evaluations of investigators. Irrespective of the prior monotherapy subgroup examined, most ophthalmologists (≥ 77%) rated the clinical effectiveness of PF tafluprost/timolol FC to be better than the prior monotherapy. Across the prior treatment subgroups, clinical signs were also considered to have improved (42.5–69.4%), or to be the similar (29.7–52.5%), when compared with prior monotherapy. These outcomes were broadly similar to those published in the primary VISIONARY study analysis [23].
The primary VISIONARY study publication reported that, although baseline CFS was typically low, the CFS score was significantly reduced for the FAS during the 6-month study period (p < 0.0001) [23]. Data in the current subanalysis for all prior PGA users showed significant reductions in CFS score following a change to PF tafluprost/timolol FC at each study visit (p ≤ 0.0186), although this appeared to have been largely driven by reductions in CFS grade within the prior bimatoprost subgroup. While numerical reductions in CFS score were observed in each prior treatment subgroup, statistically significant reductions were only observed at Week 12 and Month 6 in the prior bimatoprost subgroup. The mean (SD) baseline CFS grade was slightly higher [1.34 (1.16)] in the prior bimatoprost subgroup compared with the other groups (typically ≤ 0.97), suggesting that there was more scope for improvement during the study period in patients moving to PF tafluprost/timolol FC treatment from bimatoprost.
Although typically mild or absent at baseline, conjunctival hyperemia was significantly reduced at each study visit for prior all PGA users (p < 0.0001). These results reflect the primary VISIONARY study publication, which showed that conjunctival hyperemia was significantly reduced at Month 6, regardless of the subtype of PGA treatment used prior to switch [23]. However, our further subanalysis showed that, while prior preserved latanoprost users demonstrated a statistically significant reduction from baseline concerning conjunctival hyperemia severity, the change was not significant for those in the prior PF-latanoprost subgroup, suggesting that hyperemia may be associated with the presence of preservative agent in latanoprost users. Glaucoma patients are considered to be susceptible to ocular surface disease, and previous studies have indicated that preserved PGA formulations and some active agents may be associated with local tolerance issues [21, 30, 35,36,37,38,39,40,41]. It is well established that PGA-timolol FC therapies are associated with lower incidence of hyperemia than PGAs alone, although the exact mechanism remains undetermined [9,10,11]. Our results suggest that a switch to the PF tafluprost/timolol FC may have helped to resolve such ocular surface tolerability problems for patients included in the VISIONARY study [30, 35,36,37,38,39,40,41]. Despite overwhelming in vitro evidence, high-quality data from controlled studies are lacking regarding the potential tolerability advantages of PF medications in glaucoma therapy [35,36,37,38,39,40,41]. Further studies are required to explore whether local tolerability issues might influence the IOP-lowering efficacy of glaucoma therapies.
While real-world studies, such as the VISIONARY study, provide an important opportunity to examine the treatment outcomes achieved in routine clinical practice, this type of study is associated with a number of limitations. Patients were able to leave the study without providing a reason, and attendance at follow-up appointments also represents a major challenge for ophthalmologists in clinical practice [42]. Patients were only mandated to attend study visits at baseline and Month 6, meaning that fewer patients attended interim visits at Weeks 4 and 12, and this is reflected in the data reported at these timepoints. However, enough patients in each monotherapy subgroup attended interim visits to provide an indication of the rapidity of treatment efficacy and consistency of IOP control during the study period. Although patients treated with all formulations of PGA and beta-blocker monotherapy were allowed to enter the study, there were only sufficient patients included in the prior latanoprost group to enable further subanalysis according to whether they had used a preserved and PF formulation. Future studies would be of value to examine the impact of prior treatment with preserved or PF formulations of other PGA monotherapies on IOP-lowering efficacy, treatment adherence, and tolerability following a switch to the PF tafluprost/timolol FC. In addition, further subanalysis of VISIONARY data to explore the potential relationship between observed improvements in the signs of ocular health (e.g., hyperemia, CFS score) and IOP-lowering efficacy would be of value.
Conclusions
When switched to PF tafluprost/timolol FC treatment in a real-world setting, without a washout period, patients with OAG or OHT demonstrated clinically and statistically significant IOP reductions from baseline at Week 4, that were maintained over the 6-month study period regardless of the type of prior PGA treatment used. Improvements were observed regarding CFS score and conjunctival hyperemia, providing further evidence to support the good tolerability profile for PF tafluprost/timolol FC treatment.
Change history
22 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12325-022-02210-5
References
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505.
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40.
European Glaucoma Society. Terminology and guidelines for glaucoma 5th Edition. 2020. Available at https://www.eugs.org/eng/guidelines.asp. Accessed June 2021.
Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthal. 2002;120:1268–79.
International Council of Ophthalmology. Guidelines for glaucoma care. 2016. http://www.icoph.org/downloads/ICOGlaucomaGuidelines.pdf. Accessed October 12, 2020.
Janz NK, Wren PA, Lichter PR, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108:1954–65.
Konstas AGP, Haidich AB, Rossetti L, et al. Prostaglandin-timolol fixed combinations efficacy: myth or reality? Editorial. Eur J Ophthalmol. 2012;22:1–4.
Tabet R, Stewart WC, Feldman R, Konstas AG. A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv Ophthalmol. 2008;53(1):S85-92.
Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol. 2012;22:5–18.
Quaranta L, Biagioli E, Riva I, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29:382–9.
Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–47.
Holló G, Katsanos A, Boboridis KG, Irkec M, Konstas AG. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78(1):39–64.
Konstas AG, Katsanos A, Athanasopoulos GP, et al. Preservative-free tafluprost/timolol fixed combination: comparative 24-h efficacy administered morning or evening in open-angle glaucoma patients. Expert Opin Pharmacother. 2018;19:1981–8.
Tapply I, Broadway DC. Improving adherence to topical medication in patients with glaucoma. Patient Prefer Adher. 2021;15:1477–89.
Holló G, Hommer A, Antón López A, et al. Efficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredients. J Ocul Pharmacol Ther. 2014;30:468–75.
Holló G, Vuorinen J, Tuominen J, Huttunen T, Ropo A, Pfeiffer N. Fixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination products. Adv Ther. 2014;31(9):932–44.
Holló G, Katsanos A. Safety and tolerability of the tafluprost/timolol fixed combination for the treatment of glaucoma. Expert Opin Drug Saf. 2015;14:609–17.
Hoy SM. Tafluprost/timolol: a review in open-angle glaucoma or ocular hypertension. Drugs. 2015;75:1807–13.
Pfeiffer N, Traverso CE, Lorenz K, et al. A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components. Adv Ther. 2014;31:1228–46.
Kaarniranta K, Ikaheimo K, Mannermaa E, et al. Pharmacokinetics, efficacy, and safety of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in healthy volunteers: a phase I comparison vs. the corresponding preservative-free monotherapies. Clin Pharmacokinet. 2016;55:485–94.
Bourne RRA, Kaarniranta K, Lorenz K, Traverso CE, Vuorinen J, Ropo A. Changes in ocular signs and symptoms in patients switching from bimatoprost-timolol to tafluprost-timolol eye drops: an open-label phase IV study. BMJ Open. 2019;9(4):e024129.
Pillunat LE, Erb C, Ropo A, Kimmich F, Pfeiffer N. Preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma and ocular hypertension: results of an open-label observational study. Clin Ophthalmol. 2017;11:1051–64.
Oddone F, Tanga L, Kóthy P, Holló G. Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: the VISIONARY study. Adv Ther. 2020;37:1436–51.
Spitzer E, Cannon CP, Serruys PW. Should real-world evidence be incorporated into regulatory approvals? Expert Opin Drug Saf. 2018;9:1–5.
Eichler HG, Bloechl-Daum B, Broich K, et al. Data rich, information poor: can we use electronic health records to create a learning healthcare system for pharmaceuticals? Clin Pharmacol Ther. 2019;105(4):912–22.
Food and Drug Administration. Use of real-world evidence to support regulatory decision-making for medical devices: guidance for industry and Food and Drug Administration staff. August 31, 2018. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM513027.pdf. Accessed March 23, 2022.
Santen UK Limited. Taptiqom 15 micrograms/ml + 5 mg/ml eye drops, solution in single-dose container. Summary of product characteristics. February 2021. https://www.medicines.org.uk/emc/product/6917. Accessed March 23, 2021.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–50.
Bhapkar V. A note on the equivalence of two test criteria for hypotheses in categorical data. J Am Stat Assoc. 1966;61(313):228–35.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–23.
Holló G, Kóthy P. The Hungarian VISIONARY Study: Hungarian results in the European multicenter preservative-free tafluprost/timolol fixed combination investigation. Ophthalmologia Hungarica. 2020;157(3):196–201 (Article in Hungarian).
Ansari E, Pavicic-Astalos J, Ayan F, et al. Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: UK and Ireland Results from the VISIONARY Study. Adv Ther. 2021;38(6):2990–3002.
Karlova EV, Petrov SY, Germanova VN. Preservative-free fixed combination in the treatment of open-angle glaucoma and ocular hypertension: the VISIONARY Study (EUPAS22204). Vestn Oftalmol. 2020;136(4):76–84 (Article in Russian).
Garcia-Medina JJ, Benitez-del-Castillo J, Rodríguez-Agirretxe I, Lopez-Lopez F, Moreno-Valladares A, The VISIONARY Study Group (Spain). Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: results from the VISIONARY study population in Spain. J Ocul Pharmacol Ther. 2022;38:252–60.
Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–26.
Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279–89.
Asiedu K, Abu SL. The impact of topical intraocular pressure lowering medications on the ocular surface of glaucoma patients: a review. J Curr Ophthalmol. 2018;31(1):8–15.
Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22:730–5.
Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246:1593–601.
Garcia-Feijoo J, Sampaolesi JR. A multicentre evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–6.
Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–5.
Leiby BE, Hegarty SE, Zhan T, et al. A randomized trial to improve adherence to follow-up eye examinations among people with glaucoma. Prev Chronic Dis. 2021;18:E52.
Acknowledgements
The authors thank the participants for their valuable contribution to the VISIONARY study.
Funding
Sponsorship for this study, publication fees (Rapid Service and Open Access), and medical writing fees were provided by Santen SA. The contribution of IRCCS Fondazione Bietti to this work was supported by the Italian Ministry of Health and by Fondazione Roma.
Medical Writing and Other Assistance
Claudia Fassari, Feride Sahin and Gabriela Saborio provided input concerning the design and implementation of the study as well as the analyses and reporting of data on behalf of Santen. Claire Lea also provided input and guidance on the reporting of scientific data on behalf of Santen. Medical writing services were provided on behalf of the authors by Rebecca Down at Copperfox Communications Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Francesco Oddone, James Kirwan, Fernando Lopez-Lopez, Marina Zimina and Gábor Holló were Principal Investigators on the VISIONARY study and were responsible for the reporting of patient data, along with those authors listed in the VISIONARY study group (below). Francesco Oddone and Gábor Holló were also instrumental in the study analysis and drafting of the manuscript. Claudia Fassari was instrumental in the study design, analysis and drafting of the manuscript. Francesco Oddone, James Kirwan, Fernando Lopez-Lopez, Marina Zimina, Claudia Fassari and Gábor Holló reviewed and commented on the data reported herein and all authors approved the final manuscript.
VISIONARY Study Group Principal Investigators
Austria: Christoph Faschinger (LKH—Universitaetsklinikum Graz); Sweden: Enping Chen (St Eriks Eye Clinic, Stockholm); Hungary: Gábor Holló (Semmelweis University, Budapest), Gabor Nemeth (Borsod-Abaúj-Zemplén Megyei Kórház Szemészeti Miskolc), Gyorgy Bator (Markusovszky Egyetemi OktatóKórház, Szombathely), Alexis Tsorbatzoglou (Szabolcs-Szatmár-Bereg County Hospital and University Teaching Hospital, Nyíregyháza),Tamas Acs (Bács-Kiskun Megyei Kórház, Kecskemét), Maria Ferencz (Szent Imre Egyetemi Oktatkórház, Budapest), Zoltán Sohajda (Kenézy Gyula Kórház és Rendelőintéze, Debrecen), Jeno Toth (Fejér Megyei Szent György Egyetemi Oktató Kórház, Székesfehérvár), Veronika Volner (Uzsoki Utcai Kórház, Budapest), Gábor Vogt (Magyar Honvédség Egészségügyi Központ, Budapest), Zsolt Biro (PTE- Szemészeti Klinika, Pécs), Andrea Facskó (Szegedi Tudomá nyegyetem Szent-Györgyi Albert Klinikai Központ, Szent-Győrgy), János Nemes (Megyei Flór Ferenc Kórház, Kistarcsa), Andras Berta (University of Debrecen, Debrecen), Ilona Elek (Bugát Pál Kórház, Győngyős); Ireland: Eugene Ng (The Whitfield Clinic Butlerstown, Waterford); Italy: Francesco Oddone (IRCSS-Fondazione Bietti, Roma), Gemma Rossi (IRCCS-Fondazione Policlinico San Matteo, Pavia), Luca Rossetti (Ospedaliera San Paolo, Milan), Michele Vetrugno (Università di Bari, Bari), Michele Iester (Eye Clinic, DiNOGMI, University of Genoa, Ospedale Policlinico San Martino, Genoa, Genoa), Giorgio Marchini (Ospedale Civile Maggiore Borgo Trento, Verona), Vincenzo Scorcia (Università degli studi Magna Græcia, Catanzaro), Giovanni Staurenghi (Ospedale Luigi Sacco, Milano), Carlo Cagini (Università degli Studi di Perugia, Perugia), Tommaso Salgarello (Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma), Paolo Bettin (IRCCS Ospedale San Raffaele, Milano), Michele Figus (Ospedale Cisanello, Pisa), Gian Luca Scuderi (Ospedale Sant’Andrea, Roma), Stefano De Cilla (Azienda Ospedaliera Universitaria Maggiore della Carita Presidio Ospedaliero San Rocco, Novara); Latvia: Iveta Grundmane (Grund-opt Ltd, Valmiera), Nora Linavska (LENS-L Ltd, Liepaja), Lasma Volksone (Lavolks Ltd, Riga), Guna Laganovska (P. Stradiņš Clinical University Hospital, Riga), Kristine Baumane (Riga East Clinical University Hospital, Riga); Netherlands: Hans Lemij (Rotterdam Ophthalmic Institute, Rotterdam); Norway: Kjell Gunnar Gundersen (Dr Kjell Gunnar Gundersen MD, Haugesund); Russia: Marina Zimina (LLC Vzglyad, Leningradskaya), Valery Erichev (Federal State Budgetary Scientific Institution Scientific, Research Institute of Eye Diseases, Moscow), Elmira Adbulaeva (State Autonomous Institution of Healthcare Republican Clinical Ophthalmological Hospital of the Ministry of Healthcare of the Republic of Tatarstan, Republic of Tatarstan), Elena Karlova (State Budgetary Institution of Healthcare Samara Regional Clinical Ophthalmological Hospital, Samara), Ekaterina Zakharova (RKOB Sverdlova, Yakutsk), Irina Panova (S. Fyodorov Eye Microsurgery Federal State Institution, Saint Petersburg), Boris Malyugin (Sv. Fyodorov’s Eye Microsurgery Complex, Moscow); Spain: Iñaki Rodríguez-Agirretxe (H.U. Donostia, Guipuzcoa), Fernando Lopez-Lopez (Instituto Oftalmologico Gomez-Ulla, Galicia), Antonio Moreno Valladares (Hospital Ntra. Sra. del Perpetuo Socorro, Albacete), Javier Benitez del Castillo (Hospital Universitario de Jerez, Cadiz), Rafael Gimenez (Hospital Reina Sofia, Cordoba), Maria Parrilla Vallejo (Hospital La Macarena, Seville), Jose Javier Garcia-Medina (Hospital General Universitario Morales Meseguer, Murcia), Alfonso Anton Lopez (Institut Catalá de Retina, Barcelona), Sergio Torregrosa (Hospital Punta de Europa, Cadiz), Jorge Loscos (Hospital German Trias I Pujol, Barcelona); Denmark: Miriam Kolko (Rigshospitalet Valdemar Hansensvej, Glostrup); UK: Ejaz Ansari (Maidstone and Tunbridge Wells NHS Trust, Canterbury Christ Church University, Kent), David Broadway (Norfolk and Norwich University Hospital, Norwich, Norfolk), Katharine Claridge (Royal Cornwall Hospital, Truro, Cornwall), Simon Ruben (Southend University Hospital NHS Foundation Trust, Westcliff-On-Sea, Essex), James Kirwan (Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust, Portsmouth, Hampshire), Anca Nita (Royal Victoria Infirmary, The Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne), Michael Smith (Royal Devon & Exeter NHS Foundation Trust, Exeter, Devon), Areeb Moosavi (Milton Keynes University Hospital NHS Foundation Trust, Milton Keynes), Anthony JW King (Nottingham University Hospitals NHS Trust, Nottingham), Matthew Kinsella (Buckinghamshire Healthcare NHS Trust, Stoke Mandeville Hospital, Buckinghamshire).
Disclosures
Francesco Oddone has received consultancy fees from Santen, Allergan, Sooft, Omikron Italia and Centervue. and Novartis. James Kirwan has not received consulting fees in the last 3 years. In the last 5 years Mr Kirwan has received lecture fees from Thea, Santen and Aspire and consulting fees from Aspire and Santen. Fernando Lopez-Lopez and Marina Zimina have no relevant disclosures. Claudia Fassari is an employee of Santen SA. Gábor Holló has received consultancy and lecturing fees from Santen and Aerie.
Compliance with Ethics Guidelines
The study was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) European Union electronic Register of Post-Authorisation Studies (EU PAS Register) (EU PAS register number: EUPAS22204) and complied with the principles of the Declaration of Helsinki of 1964 as revised in 2013. All patients included were required to provide written informed consent prior to their enrolment. The study protocol was approved by the Institutional Review Board (IRB) or Independent Ethics Committee (IEC) at each center/institution and written informed consent was obtained from subjects prior to enrolment. Each of the study centers/institutions are listed in the Acknowledgments alongside the relevant Principal Investigator.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of “The VISIONARY Study Group” are listed in Acknowledgements section.
The original online version of this article was revised due to update in text.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Oddone, F., Kirwan, J., Lopez-Lopez, F. et al. Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment. Adv Ther 39, 3501–3521 (2022). https://doi.org/10.1007/s12325-022-02166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02166-6