Abstract
Introduction
The prevalence of type 2 diabetes (T2D) represents a rising burden in the US and worldwide, with the condition shown to be associated with relatively large human and economic costs. Part of the reason for such high costs associated with T2D is that the condition is often accompanied by additional health-related complications. The goal of this research is to examine the association between glycemic control and diabetes-related complications for individuals with T2D.
Methods
The Optum Clinformatics® Data Mart (CDM) database from 2007 to 2020 was used to identify adults with T2D. Individuals were classified as having sustained glycemic control (all hemoglobin A1c [HbA1c] < 7%) or poor glycemic control (all HbA1c ≥ 7%) over the 5-year post-period, and diabetes-related complications were identified based upon the Diabetes Complications Severity Index. Multivariable analyses examined the association between sustained glycemic control and diagnosis of a diabetes-related complication in the post-period.
Results
Maintaining HbA1c < 7% over the 5-year post-period, compared to maintaining HbA1c ≥ 7%, was associated with reduced odds of the diabetes-related complications of cardiovascular disease (odds ratio [OR] = 0.76, 95% confidence interval [CI] 0.61–0.94), metabolic disease (OR = 0.37, 95% CI 0.22–0.600), neuropathy (OR = 0.62, 95% CI 0.45–0.84), nephropathy (OR = 0.81, 95% CI 0.69–0.94), and peripheral vascular disease (OR = 0.52, 95% CI 0.33–0.83). There was no statistically significant association between sustained glycemic control and cerebrovascular disease.
Conclusions
Sustained glycemic control was found to be associated with significant reductions in the odds of being diagnosed with diabetes-related complications over a 5-year post-period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prevalence of type 2 diabetes (T2D) represents a rising burden in the US and worldwide, with the condition shown to be associated with relatively large human and economic costs. |
T2D is often accompanied by additional health-related complications, which have been found to be associated with morbidity and mortality. |
This study found that for individuals with T2D, maintaining hemoglobin A1c (HbA1c) < 7% over a 5-year period, compared to maintaining HbA1c ≥ 7%, was associated with reduced odds of the diabetes-related complications of cardiovascular disease, metabolic disease, neuropathy, nephropathy, and peripheral vascular disease. |
Results from this study highlight the importance of lower HbA1c for adults with T2D. |
Introduction
The prevalence of diagnosed and undiagnosed diabetes in the US population was roughly 10.5% in 2018 estimates [1]. With this relatively high prevalence and its chronic nature, it is unsurprising that diabetes has been associated with a large human and economic burden [2]. Much of this burden is due to complications [2], 3. For example, in type 2 diabetes (T2D), direct costs have been estimated to be nine times higher and health-related quality-of-life has been found to be significantly lower for those with T2D-related complications compared to individuals without such complications [3, 4]. Furthermore, all complications have been found to independently predict mortality, and macrovascular complications have been found to be the most common cause of morbidity and mortality in T2D [5,6,7].
Clinical trials have suggested that intensive glycemic control may help reduce the complications associated with T2D. For example, the United Kingdom Prospective Diabetes Study (UKPDS) found, after a median follow-up time of 10 years, that intensive drug therapy (median hemoglobin A1c [HbA1c] 7%) led to a 25% lower overall rate of microvascular complications relative to conventional treatment (median HbA1c 7.9%) [8]. While UKPDS included only people newly diagnosed with T2D and excluded individuals with markers of pre-existing vascular disease [8], later T2D clinical trials included people with long-standing diabetes and a history of, or risk factors for, vascular disease [9,10,11]. Among these later trials, the Action in Diabetes and Vascular Disease (ADVANCE) study (2008) found a statistically significant reduction in the rate of microvascular, but not macrovascular, events in an intensively treated cohort (mean HbA1c of 6.5%) relative to a standard treatment cohort (mean HbA1c of 7.3%) [10]. Similarly, in 2008 the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial observed a statistically significant reduction in nonfatal myocardial infarction associated with intensive therapy (target HbA1c of < 6.0%) compared to standard therapy (target HbA1c of 7.0% vs. 7.9%) [9].
While the above-cited studies provide some evidence of benefits associated with intensive glycemic control, T2D treatment has changed substantially since much of that research was conducted. For example, since 2005, dipeptidyl-peptidase inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have all been approved for the treatment of T2D [12]. Both GLP-1 receptor agonists and SGLT-2 inhibitors have been shown to have reno- and cardioprotective effects independent of their impact on hyperglycemia [13], while dipeptidyl-peptidase inhibitors appear to reduce cardiovascular risk factors, such as dyslipidemia and hypertension [14]. In addition, in 2008, the US Food and Drug Administration (FDA) issued new guidelines to ensure the cardiovascular safety of glucose-lowering agents (GLAs) [15].
Given the importance of T2D complications and the recent innovations in treatment, the present study examined the relationship between glycemic control, defined as sustained HbA1c < 7%, and T2D complications over a 5-year time horizon. The aim of this study was to broaden the evidence from the clinical trials as well as provide updated evidence of the association between glycemic control and diabetes-related complications. To this end, the analyses used observational data from real-world settings and included a large and geographically diverse population of individuals with T2D in the US between 2007 and 2020.
Methods
The Optum Clinformatics® Data Mart (CDM) database was licensed by Optum to Lilly and HealthMetrics for use in this study. The CDM database contains geographically diverse healthcare information for approximately 17–19 million annual covered lives from commercial health plan data and Medicare Advantage members. This information is based on the insurance claims of individuals who have both medical and prescription drug coverage as well as on the results of outpatient laboratory tests processed by large national laboratory vendors. For the present study, the CDM provided information on patient characteristics, member enrollment, outpatient services, inpatient confinements, prescription medication use, and HbA1c laboratory test results. The time horizon for all claims, tests, and encounters referenced in this study was January 1, 2007, through December 31, 2020. All data were deidentified at the patient level and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). Given the use of retrospective and deidentified data, no ethics review approval was required for this study.
To be included in the study population, individuals were required to have at least 6 years of continuous insurance coverage, as dictated by our study design, which included a 1-year pre-period and a 5-year post-period. The identification window for each person was the overlap between his or her period of continuous insurance coverage and the time horizon requirements for the pre- and post-period. For example, if an individual had continuous insurance coverage from January 1, 2010, through December 31, 2016, he or she would have an identification window of January 1, 2011, through December 31, 2011. In addition, individuals were required to have at least one recorded HbA1c within their identification window. The date of the first such HbA1c result was defined as the index date for that individual.
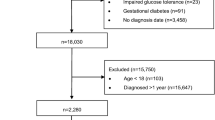
Given the above requirements, participants were included if, during the pre-period, they received at least one diagnosis of T2D and filled a prescription for at least one GLA. Individuals were excluded if they received a diagnosis of type 1 diabetes or any diabetes-related complication during the pre-period or if they were diagnosed with pregnancy or gestational diabetes at any time during the study period. Individuals younger than 18 years at index date were also disqualified. In addition, individuals were excluded if they had > 120 recorded HbA1c test results in the 5-year post-period due to an issue with the source code, which recorded an extensive number of HbA1c tests for a small subset of people. Finally, individuals were required to maintain their HbA1c either (1) below or (2) at or above 7% (the target) for the entire post-period. The choice of 7% as the threshold for glycemic control is consistent with American Diabetes Association (ADA) guidelines, which state that a HbA1c goal of < 7% is appropriate for many nonpregnant adults [16]. Figure 1 illustrates how each of the inclusion and exclusion criteria affected the sample size.
Inclusion/exclusion criteria and sample size. A: Identification window for each patient was dictated by the start and stop of their continuous enrollment period, the requirement of a 1-year pre-period, and the requirement of a 5-year post-period. The first recorded HbA1c result in the identification window was identified as the index date. B: Based upon receipt of ≥ 1 diagnosis of T2D, no receipt of any diagnoses of type 1 diabetes, and filling of at least 1 prescription for a glucose-lowering agent. C: To exclude patients with a source code that recorded a large number of HbA1c results
Outcomes examined in these analyses focused on diabetes-related complications as defined by the Diabetes Complications Severity Index (DCSI) [17, 18]. The specific complications included in the DCSI included cardiovascular disease, cerebrovascular disease, metabolic disease, neurology, nephrology, peripheral vascular disease, and retinopathy. These complications were included in the DCSI based on models and consensus from a panel of experts, including a diabetologist, an ophthalmologist, epidemiologists, nephrologists, primary care physicians, and psychiatrists [17]. Each of the DCSI complications, with the exception of retinopathy, was considered individually in this study as an outcome of interest. Retinopathy was omitted from the analyses because it was identified in < 1% of our cohort.
Descriptive statistics examined the overall cohort and also examined the unadjusted differences between individuals with sustained glycemic control, defined as sustained HbA1c < 7% over the entire post-period, and individuals with sub-optimal glycemic control, defined as sustained HbA1c ≥ 7% throughout the post-period. In addition, logistic regressions were utilized to examine the association between sustained glycemic control and the likelihood of being newly diagnosed with a diabetes-related complication. To adjust for potential underlying confounders, the multivariable analyses controlled for patient age, gender, race, region, insurance type, and pre-period adjusted Charlson Comorbidity Index (CCI) [19, 20], where factors included in both the CCI and the DCSI were omitted. Specifically, the comorbidities of myocardial infarction, congestive heart failure, peripheral vascular disease, renal disease, and diabetes with or without complications were omitted from the calculation of the adjusted CCI. The analyses also controlled for: pre-period comorbidities that are not included in the CCI but which may affect patient outcomes (anxiety, depression, and hypoglycemia); pre-period resource utilization (visits to a cardiologist, endocrinologist, neurologist, nutritionist, ophthalmologist, family practitioner, or internist); and pre-period medication use (numbers of prescribed classes of insulins, non-insulin GLAs, and non-GLA drugs).
All analyses were conducted using SAS, version 9.4. A probability value of < 0.05 was determined, a priori, to be statistically significant.
Results
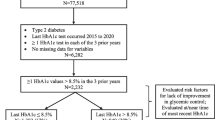
Table 1 describes characteristics for the entire cohort as well as for subgroups of individuals whose HbA1c over the post-period indicated glycemic control (HbA1c < 7%) or sub-optimal glycemic control (HbA1c ≥ 7%). There were 6037 individuals included in the study prior to requiring that HbA1c be maintained either above or below target for the entire post-period. Adding that requirement resulted in a final sample of 3067 individuals, illustrating that 50.8% of the cohort had relatively stable HbA1c over the 5-year post-period. For the 3067 individuals in the final sample, the mean age was 66.5 years, 60% were female, 54.4% were identified as white, 49.0% resided in the South, and 48.4% were insured via a health maintenance organization. Over half of those in the final sample had visited a cardiologist in the pre-period, and, on average, individuals were treated in the pre-period with 1.8 classes of GLAs and 13.1 non-GLA drugs.
The unadjusted descriptive statistics (Table 1) reveal significant differences between the people with sustained glycemic control and those with sub-optimal control. Specifically, individuals with glycemic control were significantly older, more likely to be female, less likely to be insured via a health maintenance organization, and less likely to have either visited an endocrinologist or been diagnosed with hypoglycemia in the pre-period. In addition, they had significantly more visits to a family practitioner or internist in the pre-period, and they were prescribed significantly fewer classes of insulins and non-insulin classes of GLAs.
Figure 2 shows the findings of the multivariable analyses, which are the main results of this study. As the figure reveals, individuals with sustained glycemic control, defined as all HbA1c < 7% in the post-period, compared to those with sustained sub-optimal glycemic control, defined as all HbA1c ≥ 7% in the post-period, were significantly less likely to be diagnosed in the post-period with most of the diabetes-related comorbidities of interest. In particular, maintaining HbA1c < 7% in the 5-year post-period was associated with a significantly lower likelihood of being diagnosed with: cardiovascular disease (24% lower odds, odds ratio [OR] = 0.76, 95% confidence interval [CI] 0.61–0.94), metabolic disease (63% lower odds, OR = 0.37, 95% CI 0.22–0.60), neuropathy (38% lower odds, OR = 0.62, 95% CI 0.45–0.84), nephropathy (19% lower odds, OR = 0.81, 95% CI 0.69–0.94), and peripheral vascular disease (48% lower odds, OR = 0.52, 95% CI 0.33–0.83). In contrast, cerebrovascular disease was found to have no statistically significant relationship with post-period HbA1c.
Association between sustained glycemic control (HbA1c < 7%) and the likelihood of diabetes-related complications. Results from multivariable logistic regressions which controlled for patient characteristics (age, sex, race, region, insurance type), pre-period general health and comorbidities (adjusted CCI, anxiety, depression, and hypoglycemia), pre-period resource utilization (visits to cardiologist, endocrinologist, nephrologist, nutritionist, ophthalmologist, and number of family practice/internist visits), and pre-period medication use (number of classes of insulin prescribed, number of classes of non-insulin GLAs prescribed, and number of non-GLAs prescribed). Dependent variable is sustained HbA1c < 7% (compared to sustained HbA1c ≥ 7%)
As a test of the sensitivity of results, the main analyses were repeated twice more. In the first sensitivity analysis (Fig. 3), the focus was on maintaining a more rigorous HbA1c threshold. Specifically, people were grouped based upon whether they maintained HbA1c ≤ 6.5% or > 6.5% for the entire 5-year post-period. An HbA1c target of ≤ 6.5% is consistent with the management algorithm for T2D developed by the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology. [21] The second sensitivity analysis (Fig. 4) focused on relaxing the requirement that HbA1c be maintained below 7% for the entire 5-year post-period. Instead, people were grouped based upon their HbA1c level at index date. In general, the main findings of the analyses were robust to these alternative specifications. Specifically, in both the main analyses and the sensitivity analyses, having HbA1c below target was associated with statistically significant reductions in the likelihood of being diagnosed with cardiovascular disease, metabolic disease, neuropathy, and nephropathy. However, requiring individuals to have sustained HbA1c ≤ 6.5% over the entire 5-year post-period resulted in a significant reduction in the likelihood of being diagnosed with cerebrovascular disease, while classifying individuals based upon their HbA1c at index date only revealed no statistically significant association between HbA1c and the likelihood of being diagnosed with peripheral vascular disease. It should also be noted that there was no statistically significant difference when comparing results for individuals with sustained HbA1c < 7% over the 5-year post-period compared to individuals with a HbA1c < 7% at index date. While some of the individuals in the latter group also had sustained HbA1c below target for the entire post-period, this result is consistent with research which has identified a legacy effect for long-term outcomes associated with having HbA1c below target during the first-year post-diagnosis of diabetes [22].
Sensitivity analyses examining the association between sustained HbA1c ≤ 6.5% and diabetes-related complications. Results from multivariable logistic regressions which controlled for patient characteristics, pre-period general health and comorbidities, pre-period resource, and pre-period medication use. Sample size: 3730
Sensitivity analyses examining the association between index HbA1c < 7% and diabetes-related complication results from multivariable logistic regressions which controlled for patient characteristics, pre-period general health and comorbidities, pre-period resource, and pre-period medication use. Sample size: 6037
Discussion
Microvascular Complications
Our finding that sustained glycemic control, defined as all HbA1c < 7% in the post-period, was associated with lower rates of neuropathy and nephropathy is generally consistent with the overall conclusions of UKPDS, ADVANCE, and the Veterans Affairs Diabetes Trial (VADT) clinical trials, which all noted statistically significant reductions in microvascular complications with intensive glycemic control [8, 10, 11]. Furthermore, meta-analyses of clinical trials which examined intensive glycemic control found that such control was associated with a reduction in the risk for the composite microvascular outcome [23] or the specific microvascular endpoint of nephropathy [24, 25]. The findings of reduced likelihood of being diagnosed with a microvascular complication for individuals with sustained glycemic control, defined as HbA1c < 7% throughout the post-period, are also consistent with previous nonclinical trial research. For instance, a 2011 observation of a population of Mediterranean adults newly diagnosed with T2D found that both men and women with HbA1c > 7%, either initially (mean; p < 0.001) or at follow-up (mean; p = 0.001), were more likely to develop microvascular complications sooner relative to those with lower mean HbA1c [26] . Focusing on individual microvascular complications, the present results support a Japanese multivariate analysis which found that HbA1c < 7% was associated with a decreased risk of nephropathy [27] as well as a Chinese study which concluded that HbA1c > 7.5% was one of three factors most strongly associated with a diagnosis of diabetic nephropathy [28]. Similarly, a prospective study conducted with 50 neurologically asymptomatic individuals with diabetes found that HbA1c was the most important factor predicting higher risk of subclinical neuropathy [29].
Macrovascular Complications
The results of our study suggest that glycemic control (i.e., sustained HbA1c < 7%) lowers the odds of being diagnosed with two macrovascular complications, namely, cardiovascular disease and peripheral vascular disease, over a 5-year time horizon. This result has potentially large economic implications, since macrovascular disease has been found to account for 85% of the cumulative costs of complications over a 5-year period among individuals with T2D [30]. These reductions in macrovascular complications are generally consistent with clinical trial data which examined glycemic control over an extended time horizon. Specifically, 10 years of additional observational follow-up after UKPDS revealed statistically significantly lower rates of both myocardial infarction and all-cause death among those who were initially randomized to the intensive treatment cohort [31], while 10 years of additional follow-up after VADT revealed a significant reduction in the risk of cardiovascular events in the intervention group relative to the control group [31, 32].
In contrast, the original UKPDS, ADVANCE, and VADT clinical trials all concluded that there was no statistically significant relationship between intensive antidiabetic treatment and macrovascular complications [8, 10, 11]. Meanwhile, the ACCORD trial found that the intensively treated group had a reduced risk of nonfatal myocardial infarction but also a higher rate of death, leading to early termination of the trial [9]. Inconsistencies between the present study and the original clinical trial results discussed above may reflect differences in the time horizon and populations included in these respective studies as well as changes in treatments developed for T2D and the increased emphasis on cardiovascular outcomes over the past 2 decades.
The present macrovascular findings generally support several previous non-clinical studies. A recent study of older veterans with diabetes found that increases in HbA1c time-in-range were associated with decreases in cardiovascular disease [33]. Additionally, Rawshani et al. [34] included 271,174 Swedish men and women with T2D matched with 1,355,870 controls (based on age, sex, and county) and, after a median follow-up of 5.7 years, found that HbA1c outside the target range (i.e., HbA1c ≥ 7%) was the strongest predictor of stroke and acute myocardial infarction. A smaller (n = 469) 2011 study, which looked at a Mediterranean cohort newly diagnosed with T2D, found a statistically significant association between HbA1c > 7% (at baseline and at follow-up) and an increased risk for macrovascular complications among women but not men [26]. Meanwhile, a study of Japanese individuals with T2D found that “HbA1c on target [< 7%] correlated with being free of CVD [cardiovascular disease]” [27].
Metabolic Disease
The decreased likelihood of metabolic disease among those with glycemic control in the present study is roughly consistent with previous non-clinical studies that have reported an association between lower HbA1c and a reduced rate of metabolic syndrome [35,36,37]. However, it is important to note that metabolic syndrome, defined as the presence of two or more risk factors (e.g., high blood pressure, dyslipidemia, high HbA1c, etc.) for heart disease or other problems [35,36,37], is not the same as the DCSI definition of metabolic disease. Specifically, the DCSI defines metabolic disease as any diagnosis of diabetes with ketoacidosis, hyperosmolarity, or hypoglycemia [18]. Nevertheless, both metabolic syndrome and metabolic diseases indicate serious physiological dysfunction, which has been associated with worse outcomes, including higher odds of mortality [38, 39].
Limitations
Like any research, this study has limitations. First, the analyses focused on the benefits of achieving HbA1c goals and did not examine disadvantages that may be associated with intensive treatment. For example, while some previous research has found that reductions in HbA1c are associated with cost savings [40], other research has shown that the improved health outcomes associated with intensive glycemic control may be accompanied by increases in economic costs [41]. Furthermore, while this research focuses on the HbA1c target of < 7% based upon ADA guidelines for most adults [16], there is only limited evidence suggesting that such a target may be appropriate for adults with limited life span, given the risks associated with maintaining HbA1c at such a level and the lag time associated with the benefits of such a target [42]. The analyses included only insured adults with T2D, suggesting that the results may not be generalizable to the entire US population with diabetes andhe study design precluded controlling for or studying socioeconomic status, education level, duration of diabetes, or any other characteristics that were not captured in the insurance claims. In addition, the DCSI identified < 1% of the study population as having retinopathy. This result is surprising as retinopathy may be the most common microvascular complication of diabetes [17, 43, 44]. While a strength of the current study is that individuals were followed for a relatively long time, there were no interim analyses conducted. Finally, the focus on diabetes-related complications precluded an examination of any potential cost-efficacy associated with lower HbA1c, and the study does not examine the impact of specific therapy use or treatment patterns on patient outcomes.
Conclusions
This study focused on the long-term risk of diabetes-related complications among individuals with T2D. Using real-world data, these analyses indicated that maintaining HbA1c < 7% over 5 years was associated with reduced rates of diabetes-related complications relative to having sustained HbA1c ≥ 7%. These findings illustrate some of the negative effects associated with poor glycemic control and highlights the importance of lower HbA1c for adults with T2D. Future work will examine how specific therapies and treatment patterns affect patient outcomes.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020 [Internet]. Washington DC: U.S. Department of Health & Human Services; [Updated 2020 February 11; Cited 2021 December 12]. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–27.
Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120–9.
Alzaid A, Ladrón de Guevara P, Beillat M, Lehner MV, Atanasov P. Burden of disease and costs associated with type 2 diabetes in emerging and established markets: systematic review analyses. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):785–798.
Kirkman MS, McCarren M, Shah J, Duckworth W, Abraira C. VADT Study Group. The association between metabolic control and prevalent macrovascular disease in type 2 diabetes: the VA Cooperative Study in diabetes. J Diabetes Complic. 2006;20(2):75–80.
Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–58.
Cusick M, Meleth AD, Agrón E, et al. Early Treatment Diabetic Retinopathy Study Research Group. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care. 2005;28(3):617–25.
UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
Gerstein HC, Miller ME, Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Patel A, MacMahon S, ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Blaslov K, Naranđa FS, Kruljac I, Renar IP. Treatment approach to type 2 diabetes: past, present and future. World J Diabetes. 2018;9(12):209–19.
American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S151–S167.
Papagianni M, Tziomalos K. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors. Hippokratia. 2015;19(3):195–9.
Food and Drug Administration. Guidance for industry: diabetes mellitus - evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet]. Washington DC: U.S. Department of Health and Human Services; [Updated 2008 December; Cited 2021 December 12]. https://www.fda.gov/media/71297/download.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S73–S84.
Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23.
Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-update and ICD-10 translation. J Diabetes Complic. 2017;31(6):1007–13.
D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32(5):382–7.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
American Association of Clinical Endocrinology. Type 2 diabetes glucose management goals [Internet]. Jacksonville, FL: American Association of Clinical Endocrinology; [Updated 2017; Citied 2021 December 12]. http://pro.aace.com/disease-state-resources/diabetes/depth-information/type-2-diabetes-glucose-management-goals.
Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019;42:416–26.
Hemmingsen B, Lund SS, Gluud C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898.
Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431–7.
Monami M, Candido R, Pintaudi B, Targher G, Mannucci E. Improvement of glycemic control in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31(9):2539–46.
Mata-Cases M, Prado-Lacueva CD, Salido-Valencia V, et al. Incidence of complications and mortality in a type 2 diabetes patient cohort study followed up from diagnosis in a primary healthcare centre. Int J Clin Pract. 2011;65(3):299–307.
Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care. 2016;4(1):e000294.
Lou J, Jing L, Yang H, Qin F, Long W, Shi R. Risk factors for diabetic nephropathy complications in community patients with type 2 diabetes mellitus in Shanghai: logistic regression and classification tree model analysis. Int J Health Plann Manag. 2019;34(3):1013–24.
El-Salem K, Ammari F, Khader Y, Dhaimat O. Elevated glycosylated hemoglobin is associated with subclinical neuropathy in neurologically asymptomatic diabetic patients: a prospective study. J Clin Neurophysiol. 2009;26(1):50–3.
Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25(3):476–481.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359(15):1577–89.
Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–206.
Prentice JC, Mohr DC, Zhang L, et al. Increased hemoglobin A1c time in range reduces adverse health outcomes in older adults with diabetes. Diabetes Care. 2021;44(8):1750–6.
Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–44.
Kim H, Cho J, Jang J, et al. Correlation between hemoglobin a1c and metabolic syndrome in adults without diabetes under 60 years of age. Korean J Fam Pract. 2017;7(1):60–5.
Lai Y-R, Chiu W-C, Huang C-C, et al. HbA1C variability is strongly associated with the severity of peripheral neuropathy in patients with type 2 diabetes. Front Neurosci. 2019;13:90.
Sutkovic J. Study of HbA1c as a reliable indicator for metabolic syndrome in non diabetic patients. Southeast Eur J Soft Comput. 2013;2:27–33.
Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102(6):689–92.
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43.
Lage MJ, Boye KS. The relationship between HbA1c reduction and healthcare costs among patients with type 2 diabetes: evidence from a U.S. claims database. Curr Med Res Opin. 2020;36(9):1441–7.
CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287(19):2542–51.
Munshi MN, Meneilly GS, Mañas LR, Close KL. Diabetes in aging: pathways for developing the evidence-base in clinical guidance. Lancet Diabetes Endocrin. 2020;8(10):855–67.
Chiguluri V, Cusano D, Glasheen W, Prewitt T. Segmentation of a Medicare Advantage population using the Diabetes Complications Severity Index (DCSI), 142nd American Public Health Association Annual Meeting and Exposition, 2014 November 15–19; New Orleans, LA.
Hazel-Fernandez L, Li Y, Nero D, et al. Relationship of diabetes complications severity to healthcare utilization and costs among Medicare Advantage beneficiaries. Am J Manag Care. 2015;21(1):e62-70.
Acknowledgements
Funding
Sponsorship for this study and funding for the Rapid Service and Open Access fees were provided by Eli Lilly and Company.
Editorial Asssistance
The authors would like to thank Patricia Platt (an independent contractor for HealthMetrics) for her editorial assistance in the writing and preparation of the manuscript. Ms. Platt was compensated by HealthMetrics Outcomes Research for her assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study design and conception. Maureen Lage conducted the analysis and Maureen Lage and Heather Miller had validated the analysis and wrote the first draft of the manuscript. All authors were responsible for the data curation, investigation and methodology, commented on previous versions of the manuscript and read and approved the final manuscript.
Disclosures
Kristina S Boye, Rosirene Paczkowski and Vivian T Thieu completed this research as employees and shareholders of Eli Lilly and Company. Maureen J Lage was compensated for her work on this research project by Eli Lilly and Company. Heather Miller completed this work as an intern for HealthMetrics Outcomes Research. Heather Miller is currently affiliated with the Department of Economics, Tuft University, Medford, MA, 02155.
Compliance With Ethics Guidelines
All data were deidentified at the patient level and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). Given that the study utilized retrospective and deidentified data, no ethics review approval was required. Optum licensed Clinformatics® Data Mart (CDM) to Lilly and HealthMetrics for completion of this study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to licensing restrictions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Boye, K.S., Thieu, V.T., Lage, M.J. et al. The Association Between Sustained HbA1c Control and Long-Term Complications Among Individuals with Type 2 Diabetes: A Retrospective Study. Adv Ther 39, 2208–2221 (2022). https://doi.org/10.1007/s12325-022-02106-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02106-4