Abstract
Some, but not all, intravenous iron formulations have been recognized to induce renal phosphate wasting syndrome. Most commonly this has been reported following treatment of iron deficiency anemia (IDA) with ferric carboxymaltose (FCM). A search of PubMed identified relevant randomized controlled trials (RCTs), and case studies evaluating hypophosphatemia (HPP) resulting from intravenous iron treatment. While more recent larger comparative RCTs have confirmed that the majority of patients receiving FCM, especially those with normal renal function, may experience severe HPP, complete documentation is hampered by inconsistent reporting of serum phosphate in such trials. Similarly, while case series and RCTs have documented the persistence of HPP for several weeks or even months, the lack of studies lasting beyond 5–6 weeks has constrained full understanding of the duration of effect. Clinical trials have established that the mechanism involves the bone/metabolic axis with the elevation of intact fibroblast growth factor 23 playing the central role. Reports continue to accumulate of the clinical consequences of severe HPP which are, most commonly, bone abnormalities following repetitive dosing. Case reports and studies, however, have also shown that symptomatic hypophosphatemia can occur after a single FCM dose. The frequency of such events remains unknown, in part due to lack of awareness of hypophosphatemia coupled with the fact that the most common acute symptoms of HPP (fatigue and weakness) are the same for IDA and for many of the chronic diseases that cause IDA. Changes to US and European prescribing information for FCM should raise awareness of the potential for HPP and need to monitor patients at risk for it.
Similar content being viewed by others
Some, but not all IV irons induce a renal phosphate wasting syndrome, most frequently with ferric carboxymaltose. |
Once considered both transient and clinically benign, accumulating evidence indicates that this is not the case. |
Case series and randomized controlled trials have documented that the resultant severe hypophosphatemia may persist for several weeks and even months. |
Accumulating case reports note the clinical sequelae, most notably fractures and other bone abnormalities following repeat doses; however, acute symptomatic events have occurred following a single administration. |
Recognition of these events is hampered by the fact that the most common symptoms are similarly associated with the underlying disease states; recent changes in US and European prescribing information will hopefully enhance awareness of this syndrome. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14501688.
Introduction
Hypophosphatemia was first described as a complication of intravenous (IV) iron treatment in Japanese patients receiving long-term daily treatment with IV saccharated ferric oxide [1,2,3,4]. The first case of significant and prolonged elevation of fibroblast growth factor 23 (FGF23) in a patient with IV iron-induced hypophosphatemia was reported in 2009 in New Zealand [5]. This relationship which was later confirmed in a prospective study [6]. Since these early publications, there have been numerous reports of hypophosphatemia associated with IV iron administration [7]. Multiple IV iron formulations have been implicated, including saccharated iron oxide [7] and ferric carboxymaltose (FCM) [7,8,9,10], which is responsible for most of the cases in the European Union and North America. Initially, IV iron-induced hypophosphatemia was thought to be asymptomatic, self-limited, and not associated with adverse events or clinical sequelae [10,11,12,13,14]; however, evidence is accumulating that this is not always the case [7, 15,16,17,18,19,20,21].
In their 2017 review, Zoller and colleagues elegantly outlined the then current understanding of the mechanism of iron-induced hypophosphatemia and summarized the clinical cases reported in the literature up to that point [7]. The purpose of this review is to describe recent studies on the incidence, duration, risk factors, mechanism of IV iron-induced hypophosphatemia and to catalog the expanding list of clinical cases since the 2017 review [7].
Methods
A search of PubMed identified clinical studies published from 2008 to 2020 based on comprehensive search terms for iron-deficiency anemia (IDA) and US marketed IV iron formulations. There were 20 randomized controlled trials (RCTs) that reported on serum phosphate or hypophosphatemia. Of these, 19 out of 20 evaluated FCM, five evaluated iron sucrose, and one each evaluated iron dextran and ferumoxytol. In addition nine observational, retrospective or post hoc studies were identified; all evaluated FCM, with two also reporting on iron sucrose. Fourteen case studies reporting hypophosphatemia related to IV iron treatment were also identified and included in the evaluation. An additional nine relevant papers are referenced in this review relating to hypophosphatemia, FGF23, IDA, and iron treatment. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Incidence
The majority of cases of hypophosphatemia, either symptomatic or based on serum phosphate testing, have occurred following FCM administration, with rates reported in the literature generally from approximately 40% to 70%. However, a recent systematic review of the literature for the past 10 years showed that only 32% (20 of 63) of RCTs investigating IV iron formulations marketed in the USA for IDA reported on serum phosphate and/or hypophosphatemia [9]. Further, it was noted that the methods for measurement and reporting of serum phosphate and hypophosphatemia were highly variable, even among current prospective trials. The absence of standard protocols for assessing and monitoring phosphate levels, and variability in the reporting, definitions, and follow-up of hypophosphatemia make it difficult to assess the true incidence of this syndrome. It is likely that inconsistent assessment of hypophosphatemia has contributed to an underestimation of its actual prevalence, clinical significance, and the general lack of awareness of hypophosphatemia among clinicians.
Recent clinical trials paint a clearer picture. The FIRM trial was one of the largest (N = 1997) head-to-head phase 3 randomized, double-blind, controlled trials of IV iron formulations. It compared rates of hypersensitivity reactions, including anaphylaxis, in response to a single course of FCM (two doses of 750 mg administered 1 week apart) or ferumoxytol (two doses of 510 mg administered 1 week apart) [22]. A pre-specified secondary outcome was the incidence of, and clinical risk factors for, hypophosphatemia. The incidence of severe hypophosphatemia (serum phosphate < 2.0 mg/dL) and extreme hypophosphatemia (serum phosphate < 1.3 mg/dL) was significantly higher in patients receiving FCM versus ferumoxytol (severe: 50.8% versus 0.9%; extreme: 10.0% versus 0.0%; P < 0.001 for each) [10]. Recently reported results of pooled data from two identical randomized studies (the PHOSPHARE trials) that compared FCM (two doses of 750 mg administered 1 week apart) to ferric derisomaltose (one dose of 1000 mg) in patients with IDA also noted a significantly higher rate of hypophosphatemia among patients treated with FCM versus those treated with ferric derisomaltose (74.4% versus 8.0%; P < 0.001). Severe hypophosphatemia (≤ 1.0 mg/dL) was not observed in any patient treated with ferric derisomaltose, but developed in 11.3% of FCM-treated patients [23].
An unanswered question is why the incidence of hypophosphatemia is markedly higher with certain IV iron formulations versus others. However, so far, no studies have elucidated the mechanism.
Duration of Effect
Although initial investigations suggested that hypophosphatemia following treatment with FCM typically lasted for 2–3 weeks, recent studies have demonstrated that it can be substantially longer. In the FIRM trial, 29.1% of patients treated with FCM remained hypophosphatemic at the end of the 5-week study period, including 4.7% with extreme hypophosphatemia, compared with none of the ferumoxytol-treated patients [10]. Likewise, hypophosphatemia persisted at day 35 in 43.0% of FCM-treated patients in the trials that compared FCM to ferric derisomaltose [23]. Similar results have been observed outside of RCTs. In a chart review of 130 patients treated with either iron sucrose (mean dose 701 mg) or FCM (mean dose 2123 mg) between January 2012 and December 2014 the mean duration of hypophosphatemia in the iron sucrose group was between 2 and 18 weeks, while the mean duration in the FCM group was 6 months, with some patients never reaching normal phosphate levels during the 2-year study period [24].
The persistence of IV iron-induced hypophosphatemia is illustrated by two patients from a 2014 case series [25]. Both developed hypophosphatemia (one had a serum phosphate levels 0.25 the other 0.28 mmol/L) after infusions of FCM. Both were treated orally or intravenously with phosphate and their phosphate levels improved or normalized. However, their phosphate levels declined again such that one patient had a phosphate level of 0.17 mmol/L 3 days later requiring further phosphate treatment. The other patient had persistent hypophosphatemia (0.2 mmol/L) despite being on oral phosphate therapy and required two IV courses of phosphate to normalize her levels over the next 7 weeks. Her phosphate levels remained low over the subsequent month, despite continued oral phosphate and cholecalciferol treatment [25]. The mechanism for the recurrent/persistent hypophosphatemia demonstrated by these cases despite treatment and “temporary” improvement of serum levels remains unknown; however, it likely involves persistent increase in FGF23 and parathyroid hormone (PTH). Clinically, the cases suggest the need for the monitoring of serum phosphate levels after IV iron supplements, at least in situations of severe hypophosphatemia for at least a month or two after termination of IV iron treatment.
Hindering a more complete understanding of the usual duration of hypophosphatemia has been the fact that almost all the controlled prospective trials are relatively short (ca. 35 days) [22, 23, 26, 27]. In these studies, a high percentage of the FCM patients had severe hypophosphatemia at the end of the 5-week follow-up. It is unknown how much longer it would have persisted if surveillance continued. In an observational 6-week study of patients with inflammatory bowel disease after a single 1000 mg FCM dose, 56.9% of patients had moderate-to-severe hypophosphatemia at week 2 and 13.7% of patients still had it at week 6 [26]. The case reports and chart reviews highlight the major unmet medical need for well-controlled, longer-term data on the duration of hypophosphatemia following treatment. In concert, more data are needed on patients who receive repeated courses of FCM treatment. This includes pre and post levels of FGF23 and markers of bone metabolism such as bone alkaline phosphatase.
Another limitation in the available dataset is the absence of trials that prospectively and systematically collected and reported data specifically pertaining to adverse clinical sequelae of hypophosphatemia. These were not reported in the FIRM or PHOSPHARE trials, leaving open the question regarding whether and to what extent they occur, when significant hypophosphatemia follows iron infusion. The adverse sequelae of hypophosphatemia were not specifically sought in these trials, although any and all adverse events were collected and adjudicated. While the incidence of hypophosphatemia secondary to FCM was elevated, the rate of symptomatic events is likely less and may be undetected in cohorts of this size.
Pathophysiologic Mechanisms of IV Iron-Induced Hypophosphatemia
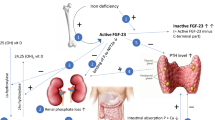
Working through multiple feedback loops, PTH, 1,25-dihydroxyvitamin D [1,25(OH)2D], and FGF23 regulate serum phosphate by modulating intestinal phosphate absorption, renal phosphate reabsorption, and bone metabolism [28]. Malfunction of these feedback loops may result in several diseases. Primary increases in FGF23 cause hypophosphatemia and suppression of 1,25(OH)2D, which leads to rickets or osteomalacia. Secondary increases in FGF23 are one of the earliest indicators of declining renal function, and the magnitude of FGF23 elevation is strongly associated with and may contribute to increased future risk of cardiovascular disease and death [29,30,31,32,33,34,35,36,37,38,39,40,41].
The role of 1,25(OH)2D in IV iron-induced hypophosphatemic-related osteomalacia was reported as early as 1997 [3]. It was not until 2009, however, that the role of FGF23 in mediating phosphate wasting was first proposed [6]. The bone/metabolic axis and the interplay with iron deficiency and treatment of IDA with IV iron formulations was further elucidated in a randomized study of 55 women with IDA caused by abnormal uterine bleeding who were treated with either FCM or iron dextran. The study hypothesized that some IV iron formulations induce renal phosphate wasting by inhibiting the degradation or cleavage of FGF23, thus leading to an increase in intact FGF23 [26]. The study evaluated the association of IDA with plasma measurements of both intact FGF23 (iFGF23) and C‐terminal FGF23 (cFGF23). The iFGF23 assay detects full-length biologically active FGF23 exclusively; the cFGF23 assay detects both the full-length peptide, but also its C-terminal cleavage fragments. Iron deficiency stimulates increased transcription of FGF23, but this is balanced by an increase in cleavage of the FGF23. The net result is marked elevations of cFGF23 (due to elevated levels of C-terminal fragments), but normal iFGF23 levels and thereby normal serum phosphate levels. At baseline, the iron-deficient subjects had markedly increased levels of cFGF23. Both treatments led to an 80% decline in cFGF23 within 24 h. Treatment with FCM, but not iron dextran, was associated with a significant increase in intact FGF23 levels within 24 h, a subsequent increase in urinary fractional excretion of phosphate (FEPi), and decreases in serum phosphate, 1,25(OH2)D, and calcium levels, and also increases in PTH [27]. These results confirmed and further elucidated the previous reports of acute FGF23-mediated phosphate wasting after treatment with IV iron formulations [1,2,3,4, 6].
Recent randomized clinical trials have confirmed that increased FGF23 activates the cascade leading to hypophosphatemia after treatment with FCM. Results of a pre-planned secondary analysis from the FIRM trial showed that FCM, but not ferumoxytol, rapidly increased iFGF23 levels [10]. Similarly, the pooled analysis of the two separate but identical PHOSPHARE trials also showed increased intact FGF23 levels with FCM, increased urinary FEPi, decreased serum 1,25(OH)2D, decreased ionized calcium, and increased PTH (Fig. 1). This study extended our understanding of the interplay of these hormones and factors with bone metabolism by demonstrating significant elevations in biomarkers of bone turnover (Fig. 1i) that are associated with osteomalacia [23].
LS mean changes from baseline in biomarkers of mineral and bone homeostasis according to iron treatment. Red arrows indicate infusion of ferric carboxymaltose, 750 mg; blue arrows indicate infusion of iron isomaltoside, 1000 mg. *P < 0.05, **P < 0.01, ***P < 0.001 between-group comparisons from a mixed model for repeated measures analysis with treatment day, treatment-by-day, trial and stratum as fixed effects and baseline value and baseline value-by-day as covariates; safety analysis set. FCM, ferric carboxymaltose; FGF23, fibroblast growth factor 23; IIM, iron isomaltoside 1000/ferric derisomaltose; LS, least squares; SE, standard error. Reproduced with permission from JAMA. 2020;323(5):432–443. Copyright©(2020) American Medical Association. All rights reserved.
Risk Factors
Two recent studies have attempted to identify which patients may be at greatest risk for hypophosphatemia [10, 42]. The first was a prospective, observational study designed to describe the change in FGF23, serum phosphate, and markers of bone and iron metabolism and to identify clinical and biochemical predictors of low serum phosphate after a single IV infusion of FCM (1 g). The study population (N = 65) included three groups of women with iron deficiency: pregnant women in their second or third trimester with no renal abnormalities, women with stage 3a–4 non-dialysis-dependent chronic kidney disease (CKD), and a group of controls comprised of women with menorrhagia or gastrointestinal sources of iron loss with normal renal function. Following treatment with FCM, iFGF23 increased 2.8–5.4-fold. FEPi increased in all cohorts with the greatest increase in those with CKD, with decreases in phosphate levels of 56–76%. Key predictors of serum phosphate decline were low baseline phosphate level and weight-adjusted iron dose (FCM/kg). Of interest, although at nadir the phosphate level remained higher than with the other two groups, the percentage decrease in phosphate and increase in FEPi following FCM was greatest in those with CKD. Thus, despite the kidney having impaired ability to excrete phosphate, FCM was able to dramatically increase clearance further [42].
The second study was a preplanned multivariable analysis of data from the FIRM trial, which showed that treatment with FCM versus ferumoxytol was the strongest independent risk factor for incident hypophosphatemia (odds ratio [OR] 250.6, 95% CI 115.4–544.5) [10], followed by the presence or absence of CKD. Baseline serum phosphate, abnormal uterine bleeding as the etiology of IDA, and lower body weight were also independent risk factors. Other risk factors included higher estimated glomerular filtration rate (eGFR) and hemoglobin. CKD as the etiology of IDA was associated with lower risk. The effects of lower body weight and serum phosphate strengthened as risk factors for persistent hypophosphatemia, while black race emerged as an additional independent risk factor [10].
These reports suggest a number of factors that appear to increase risk. The most important is which IV iron formulation is administered, with by far the highest incidence of hypophosphatemia occurring with FCM administration. Second most important is whether or not the patient has renal dysfunction [7, 10, 42]. Mechanistically this should not be a surprise since the inability to clear phosphate is well recognized as an issue in patients with advancing kidney disease, although in the FIRM study even those with CKD (21.5%) developed severe hypophosphatemia. This was far less than the 64% rate in those without CKD [10], but still significant, demonstrating that the reduced ability to clear phosphate in CKD is not absolute protection. Some risk factors are perhaps less intuitive. As noted, the impact of body weight was demonstrated in both the single-arm study of three different cohorts [42] and the large comparative study of ferumoxytol vs FCM [10]. Since the patients in both studies received a fixed dose of FCM, the mg/kg dose was higher with lower body weight. Although “lower dose” may attenuate risk, it should be noted that hypophosphatemia had occurred following the first 750 mg of FCM in a double-blind RCT [22] and has resulted in symptomatic events even after a 500 mg dose [15]. Not surprisingly, the lower the baseline phosphate, the greater the likelihood of hypophosphatemia [10, 42]. Finally, and perhaps most hypothesis generating, is that women with abnormal uterine bleeding as the cause of their IDA are more likely to develop hypophosphatemia [10]. Whether that is due to their being younger with perhaps better preserved renal function, or due to their iron deficiency being inadequately addressed for an extended period, or some other factor, is unknown.
Patients with chronic recurrent blood loss such as occurs with abnormal uterine bleeding or inflammatory bowel disease may have chronic and severe iron deficiency that can stimulate FGF23 transcription. Not only may this place such patients at greater risk of acute hypophosphatemia but, when coupled with repeat dosing of FCM which causes prolonged inhibition of FGF23 cleavage, it may make them a greater risk for osteomalacia and other bone abnormalities [7, 27].
New Cases of Symptomatic Hypophosphatemia
Since the 2017 review [7] a number of new case reports of iron-induced hypophosphatemia have emerged. Table 1 presents the salient features of the reports. These can perhaps be categorized as those presenting as bone abnormalities secondary to repeat courses of treatment, as were the earliest reports of symptomatic hypophosphatemia following IV iron treatment, and more acute presentations after a single course.
Several reports note severely symptomatic musculoskeletal sequelae after repeated dosing with IV iron formulations, most commonly FCM. As noted, almost all occurred following repetitive dosing for a year or longer, resulting in not only osteomalacia but generally radiologic evidence of fractures (Fig. 2).
Patient diagnosed with symptomatic hypophosphatemia and osteomalacia with bilateral symmetric pseudofractures (looser zones) in the femur necks. a Magnetic resonance imaging showing marked hyperintensities of both femoral necks on T1-weighted imaging using a turbo inversion recovery magnitude sequence. Hyperintensities mark horizontal hypointensities extending halfway across the femoral neck. b X-ray plain film radiograph fails to show the fracture lines. Reprinted from Gastroenterology, 152(6), Benedikt Schaefer, Bernhard Glodny, Heinz Zoller, Blood and Bone Loser, e5–e6, Copyright (2017), with permission from Elsevier
Although the majority of bone involvement due to repetitive IV dosing with iron has occurred in patients with Crohn’s disease, it has been reported with other gastrointestinal disorders with chronic or recurrent bleeding, such as gastric antral vascular ectasia or hereditary hemorrhagic telangiectasia.
Clinically significant hypophosphatemia following single or shorter courses of IV iron formulations are less common but have been reported. One woman developed tiredness, diffuse muscle pain, and weakness after being treated twice with a single course of FCM (500 mg) 3 weeks prior to her presenting with her symptoms. Laboratory tests revealed severe (0.23 mmol/L) hypophosphatemia [15]. Another patient presented with increasing fatigue and shortness of breath 3 weeks after finishing a course of FCM (dose not stated but assumed to be the indicated two doses of 750 mg, since was reported from the USA). Her phosphate level was 1.2 mg/dL. Despite IV and oral treatment with phosphate, serum phosphate remained critically low (0.72 mg/dL) and she developed respiratory failure. Eventually symptoms resolved after IV administration of phosphate and calcitriol [21].
Recognition of symptomatic hypophosphatemia is challenged by the fact that the most common symptoms of acute hypophosphatemia, fatigue and weakness, are also the symptoms most commonly reported for IDA and for many of the chronic diseases that cause IDA [43, 44]. Hypophosphatemia is not always considered as a source of ongoing fatigue, which instead is attributed to the underlying disease or to anemia despite evidence of resolution of the IDA. In a retrospective review of patients with hypophosphatemia who received either FCM or iron sucrose, 55% reported improvement in fatigue symptoms. However, 30% complained of fatigue worsening, and the remainder reported no change in fatigue presumably because of severe hypophosphatemia since hemoglobin levels had been corrected [24]. The diagnosis of iron-induced hypophosphatemia requires a high level of alertness and suspicion and is based on recognizing its temporal association with a newly started IV iron therapy [7].
Discussion
This review of renal phosphate wasting following IV iron treatment has emphasized a number of points. Larger, prospective trials have confirmed that the incidence of severe hypophosphatemia is very high following administration of certain IV iron formulations; in those with normal renal function the majority of patients who are treated will experience a severe drop in serum phosphate. Comparative studies establish that almost exclusively this occurs following FCM with negligible rates following iron dextran or ferumoxytol and a low but not negligible incidence with iron sucrose or iron isomaltose (ferric derisomaltose). Although the peak occurrence of hypophosphatemia is at about 2–3 weeks post dose, more recent trials establish that a large percentage of patients continue to manifest severe hypophosphatemia weeks and even months after the last dose of the iron treatment. The larger, more recent trials have provided some understanding of patients who have heightened risk for the development of severe hypophosphatemia. In general, and not surprisingly, this occurs much more commonly in patients with relatively normal renal function. Future understanding of the duration of effect as well as the time course of the overall phenomenon will require well-controlled trials that extend beyond the current 5–6 weeks.
Most importantly, symptomatic cases continue to accumulate. Although most usually occur after repetitive dosing, it is also clear that symptoms can occur following even a single dose. Recognition of the latter is hampered by the fact that the most common symptom is fatigue, often inappropriately attributed to either the underlying causative condition for the IDA or to persistence of anemia.
Contemporaneous to the preparation of this review are actions by regulatory authorities that highlight the importance of this phenomenon and which, hopefully, will enhance the awareness of it by clinicians. Presumably, these were driven by recognition of accumulating symptomatic cases. The US prescribing information for FCM (brand name Injectafer in the USA) had previously noted the incidence of hypophosphatemia and described a single case. However, in mid-February 2020, the US Food and Drug Administration updated the “warning and precautions” section for FCM to acknowledge that “symptomatic hypophosphatemia requiring clinical intervention has been reported in patients at risk of low serum phosphate. These cases have occurred mostly after repeated exposure to FCM in patients without a history of renal impairment.” The updated label also notes that healthcare providers should “monitor serum phosphate levels in patients at risk for hypophosphatemia who require a repeat course of treatment”. The Australian Department of Health has also issued a safety alert to healthcare providers, noting that FCM (marketed as Ferinject in Australia) is known to cause hypophosphatemia that is usually mild and asymptomatic. They go on to state, however, that it is also associated with a rare risk of severe symptomatic hypophosphatemia and echo earlier recommendations in the literature that healthcare providers “routinely evaluate patient risk factors before commencing Ferinject and follow up at-risk patients” [45]. Finally and most recently, the European Medicines Agency modified the language required from “parenteral administered iron preparations can cause hypophosphatemia which in most cases is transient and without clinical symptoms” to “Symptomatic hypophosphatemia leading to osteomalacia and fractures requiring clinical intervention including surgery has been reported in the post marketing setting. Patients should seek medical advice if they experience worsening fatigue with myalgias or bone pain. Serum phosphate should be monitored in patients who receive multiple administrations at higher doses or long-term treatment, and those with existing risk factors. In cases of persisting hypophosphatemia, treatment with ferric carboxymaltose should be re-evaluated.”
Conclusions
This review emphasized that if clinicians are unaware of the occurrence and importance of hypophosphatemia they are unlikely to appropriately evaluate their patients, so the authors urge clinicians to retrain their thinking to match the motto, “if you don’t look you will never find” and to monitor serum phosphate levels, especially when using FCM.
References
Okada M, Imamura K, Fuchigami T, et al. 2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long-term intravenous administration of saccharated ferric oxide. Nihon Naika Gakkai Zasshi. 1982;71(11):1566–72.
Okada M, Imamura K, Iida M, Fuchigami T, Omae T. Hypophosphatemia induced by intravenous administration of saccharated iron oxide. Klin Wochenschr. 1983;61(2):99–102.
Sato K, Nohtomi K, Demura H, et al. Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone. 1997;21(1):57–64.
Sato K, Shiraki M. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J. 1998;45(4):431–9.
Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009;46(Pt 2):167–9.
Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–7.
Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266–75.
Emrich IE, Lizzi F, Seiler-Mußler S, et al. Hypophosphatemia after high dosage iron substitution with ferric carboxymaltose (FCM) and iron isomaltoside (IM)—the randomised controlled Home Afers 1 trial. Blood. 2018;132(Suppl 1):3627.
Glaspy JA, Lim-Watson MZ, Libre MA, et al. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Ther Clin Risk Manag. 2020;16:245–59.
Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23):e124486.
Barish CF, Koch T, Butcher A, Morris D, Bregman DB. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: two randomized, controlled trials. Anemia. 2012;2012:172104.
Grimmelt AC, Cohen CD, Fehr T, Serra AL, Wuethrich RP. Safety and tolerability of ferric carboxymaltose (FCM) for treatment of iron deficiency in patients with chronic kidney disease and in kidney transplant recipients. Clin Nephrol. 2009;71(2):125–9.
Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–607.
Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719–28.
Anand G, Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep. 2017;2017:bcr2016219160.
Bartko J, Roschger P, Zandieh S, Brehm A, Zwerina J, Klaushofer K. Hypophosphatemia, severe bone pain, gait disturbance, and fatigue fractures after iron substitution in inflammatory bowel disease: a case report. J Bone Miner Res. 2018;33(3):534–9.
Bishay RH, Ganda K, Seibel MJ. Long-term iron polymaltose infusions associated with hypophosphataemic osteomalacia: a report of two cases and review of the literature. Ther Adv Endocrinol Metab. 2017;8(1–2):14–9.
Fang W, McMahon LP, Bloom S, Garg M. Symptomatic severe hypophosphatemia after intravenous ferric carboxymaltose. JGH Open. 2019;3(5):438–40.
Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018. https://doi.org/10.1136/bcr-2017-222851.
Urbina T, Belkhir R, Rossi G, et al. Iron supplementation-induced phosphaturic osteomalacia: FGF23 is the culprit. J Bone Miner Res. 2018;33(3):540–2.
Vasquez-Rios G, Marin E, Martin K, Merando A. Harder to breathe: an unusual case of severe hyperphosphaturic hypophosphatemia and normal FGF-23 levels in a young female patient [abstract 314]. Am J Kidney Dis. 2018;71(4):594.
Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol. 2018;93(5):683–90.
Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–43.
Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol. 2015;2015:468675.
Blazevic A, Hunze J, Boots JM. Severe hypophosphataemia after intravenous iron administration. Neth J Med. 2014;72(1):49–53.
Detlie TE, Lindstrom JC, Jahnsen ME, et al. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment Pharmacol Ther. 2019;50(4):397–406.
Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–803.
Fukumoto S. Phosphate metabolism and vitamin D. Bonekey Rep. 2014;3:497.
Arnlov J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83(1):160–6.
Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84(4):776–85.
Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408.
Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92.
Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–52.
Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8.
Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–9.
Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–7.
Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640–8.
Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24(1):125–35.
Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. 2014;10(5):268–78.
Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–60.
Wolf M. Mineral (mal)adaptation to kidney disease-Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol. 2015;10(10):1875–85.
Huang LL, Lee D, Troster SM, et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dial Transplant. 2018;33(9):1628–35.
Strauss WE, Auerbach M. Health-related quality of life in patients with iron deficiency anemia: impact of treatment with intravenous iron. Patient Relat Outcome Meas. 2018;9:285–98.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23.
Suzuki K, Suzuki S, Ishii Y, et al. Plasma prostaglandin D2 synthase levels in sleep and neurological diseases. J Neurol Sci. 2020;411:116692.
Schaefer B, Glodny B, Zoller H. Blood and bone loser. Gastroenterology. 2017;152(6):e5–6.
Reyes M, Diamond T. Hypophosphataemic rickets due to parenteral ferrous carboxymaltose in a young man with crohn disease and iron deficiency: a case report and review of literature. J Clin Case Rep. 2017;07(02):931.
Gomez Rodriguez S, Castro Ramos JC, Abreu Padin C, Gómez Peralta F. Intravenous iron induced severe hypophophatemia in a gastric bypass patient. Endocrinol Diabetes Nutr. 2019;66(5):340–2.
Chen YJ, Lim C, McCormick J. Resistant iron-induced hypophosphatemia following colorectal surgery. N Z Med J. 2019;132(1499):72–5.
Roman-Gimeno S, Ortez-Toro JJ, Peteiro-Miranda CM, Sanz-Martin B, Urdaniz-Borque R. Case report: a rare cause of severe hypophosphatemia. Ann Endocrinol (Paris). 2020;81(2–3):125–6.
Sullivan A, Lanham T, Rubin A. A rare case of parental iron-induced persistent hypophosphatemia. J Commun Hosp Intern Med Perspect. 2020;10(2):166–7.
Callejas-Moraga EL, Casado E, Gomez-Nuñez M, Caresia-Aroztegui AP. Severe osteomalacia with multiple insufficiency fractures secondary to intravenous iron therapy in a patient with Rendu-Osler-Weber syndrome. Bone Rep. 2020;13:100712.
Acknowledgements
Funding
Funding for the journal’s Rapid Service and Open Access Fees Was provided by AMAG Pharmaceuticals Inc. The authors received no payment for their work.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance was provided by Maria B. Vinall of The Curry Rockefeller Group, LLC, Tarrytown, NY, USA, and this assistance was funded by AMAG Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
JAG, WES, and MW each participated in designing and conceiving the review, contributed to each draft of the manuscript and reviewed the final draft of the paper and approved its final submission.
Disclosures
John A. Glaspy has served as a consultant to AMAG Pharmaceuticals Inc. and Luitpold Pharmaceuticals Inc. Myles Wolf has served as a consultant to AMAG Pharmaceuticals Inc., Luitpold Pharmaceuticals Inc., and Pharmacosmos, Inc. William E. Strauss was a full-time employee and held equity in AMAG Pharmaceuticals Inc. at the time of the initial drafting of this manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Glaspy, J.A., Wolf, M. & Strauss, W.E. Intravenous Iron-Induced Hypophosphatemia: An Emerging Syndrome. Adv Ther 38, 3531–3549 (2021). https://doi.org/10.1007/s12325-021-01770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01770-2