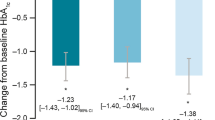Abstract
Introduction
The IMPROVE™ study is an openlabel, nonrandomized, observational study aimed at determining the safety and efficacy of biphasic insulin aspart 30 (BIAsp 30) treatment in subjects with type 2 diabetes from 11 countries. Here, we report the baseline data of the Indian cohort.
Methods
All subjects with type 2 diabetes requiring insulin and considered suitable for BIAsp 30 therapy based on their physician’s clinical judgment were eligible to enter the study. The data recorded at baseline included demographic characteristics, detailed medical histories, physician-cited reasons for starting BIAsp 30 treatment, and the chosen dosage regimens.
Results
The Indian cohort included 17,995 subjects with diabetes. Poor glycemic control (glycated hemoglobin [HbA1c], 8.7%–9.6%) was observed at baseline in all four geographical zones (North, South, East, and West) and prestudy treatment groups (no therapy, only oral antidiabetic drug [OAD], OAD ± insulin, and OAD ± insulin ± BIAsp 30). Prevalence of both micro- and macrovascular complications was high, also reflecting poor glycemic control. Improving HbA1c and fasting and postprandial blood glucose levels were the most common reasons for starting BIAsp 30 therapy. The subjects were prescribed a mean BIAsp 30 dose of approximately 24 IU, and a twice-daily regimen was employed in almost 80% of subjects.
Conclusion
The baseline results of the IMPROVE study Indian cohort confirm the poor glycemic control and the delayed initiation and/or inadequacy of treatment in subjects with type 2 diabetes. These results also highlight the need for timely and appropriately intensive insulin-based therapy.
Similar content being viewed by others
References
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412.
Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–1309.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
European Diabetes Policy Group. A desktop guide to type 2 diabetes mellitus. Diabet Med. 1999;16:716–730.
Bebakar WMW, Chow CC, Kadir KA, et al. Adding biphasic insulin aspart 30 once or twice daily is more efficacious than optimizing oral antidiabetic treatment in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:724–732.
Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes. 2006;114:527–532.
Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265.
Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complications. 2003;17:307–313.
Yang W, Ji Q, Zhu D, et al. Biphasic insulin aspart 30 three times daily is more effective than a twicedaily regimen without increasing hypoglycemia in Chinese subjects with type 2 diabetes inadequately controlled on oral antidiabetes drugs. Diabetes Care. 2008;31:852–856.
Ligthelm RJ, Borzi V, Gumprecht J, Kawamori R, Wenying Y, Valensi P. Importance of observational studies in clinical practice. Clin Ther. 2007;29:1284–1292.
Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218.
Sharma SK, Joshi SR, Kumar A, et al. Efficacy, safety and acceptability of biphasic insulin aspart 30 in Indian patients with type 2 diabetes: results from the PRESENT study. J Assoc Physicians India. 2008;56:859–863.
Valensi P, Benroubbi M, Borzi V, et al. The IMPROVE T Study — a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008:62;1809–1819.
American Diabetes Association. Standards of medical care in diabetes — 2008. Diabetes Care. 2008;31(suppl. 1):S12–S54.
Raheja BS, Kapur A, Bhoraskar A, et al. DiabCare Asia-India Study: diabetes care in India-current status. J Assoc Physicians India. 2001;49:717–722.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053.
Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-1. Invest Ophthalmol Vis Sci. 2005;46:2328–2333.
Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55). Diabet Med. 2008;25:407–412.
Mohan V, Deepa R, Shanthirani CS, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in south India. J Am Coll Cardiol. 2001;38:682–687.
Premalatha G, Shanthirani CS, Deepa R, Markovitz J, Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected south Indian population — the Chennai Urban Population Study (CUPS). Diabetes Care. 2000;23:1295–1300.
Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycaemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356.
Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (the 1-2-3 study). Diabetes Obes Metab. 2006;8:58–66.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S., Das, A.K., Kumar, A. et al. Baseline characteristics of the Indian cohort from the IMPROVE™ study: a multinational, observational study of biphasic insulin aspart 30 treatment for type 2 diabetes. Adv Therapy 26, 325–335 (2009). https://doi.org/10.1007/s12325-009-0006-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-009-0006-9