Abstract
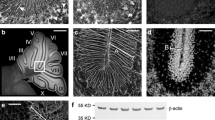
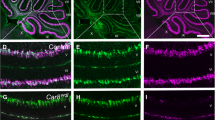
The Acp2 gene encodes lysosomal acid phosphatase 2 (ACP2), an isoenzyme that hydrolyzes orthophosphoric monoesters to alcohol and phosphate. Mutations in this gene compromise lysosomal function and cause acid phosphatase deficiency. Loss of Acp2 in the brain causes defects in the cerebellum. Here, we performed an in-depth protein expression analysis in the mouse cerebellum to understand how Acp2 controls cellular function in the developing and adult brain. We have found that during development, ACP2 expression marks the caudal midbrain and cerebellum, two regions that are linked by multiple signaling mechanisms during embryogenesis. By around P8, ACP2 was localized predominantly to the somata of Purkinje cells, the principal neurons of the cerebellar cortex. During the second postnatal week, we found that ACP2 expression expanded into the dendrites and axon terminals of Purkinje cells. However, at 2 weeks of age, only a subset of Purkinje cells strongly express ACP2. Further expression analyses revealed that in the mature cerebellum, ACP2 expression divided Purkinje cells into a pattern of molecular zones that are associated with the functional topography of sensory-motor circuitry. These data suggest that ACP2 expression is dynamically regulated during development, and in the adult, it may function within a complex architecture that is linked to cerebellar modular organization.






Similar content being viewed by others
References
Makrypidi G, Damme M, Muller-Loennies S, Trusch M, Schmidt B, Schluter H, et al. Mannose 6 dephosphorylation of lysosomal proteins mediated by acid phosphatases Acp2 and Acp5. Mol Cell Biol. 2012;32:774–82.
Coutinho MF, Prata MJ, Alves S. Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction. Mol Genet Metab. 2012;105:542–50.
Lubke T, Lobel P, Sleat DE. Proteomics of the lysosome. Biochim Biophys Acta. 2009;1793:625–35.
Bagshaw RD, Mahuran DJ, Callahan JW. Lysosomal membrane proteomics and biogenesis of lysosomes. Mol Neurobiol. 2005;32:27–41.
Schroder BA, Wrocklage C, Hasilik A, Saftig P. The proteome of lysosomes. Proteomics. 2010;10:4053–76.
De Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955;60:604–17.
Mannan AU, Roussa E, Kraus C, Rickmann M, Maenner J, Nayernia K, et al. Mutation in the gene encoding lysosomal acid phosphatase (Acp2) causes cerebellum and skin malformation in mouse. Neurogenetics. 2004;5:229–38.
Gottschalk S, Waheed A, Schmidt B, Laidler P, von Figura K. Sequential processing of lysosomal acid phosphatase by a cytoplasmic thiol proteinase and a lysosomal aspartyl proteinase. EMBO J. 1989;8:3215–9.
Hille A, Klumperman J, Geuze HJ, Peters C, Brodsky FM, von Figura K. Lysosomal acid phosphatase is internalized via clathrin-coated pits. Eur J Cell Biol. 1992;59:106–15.
Braun M, Waheed A, von Figura K. Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J. 1989;8:3633–40.
Geier C, Kreysing J, Boettcher H, Pohlmann R, von Figura K. Localization of lysosomal acid phosphatase mRNA in mouse tissues. J Histochem Cytochem: Off J Histochem Soc. 1992;40:1275–82.
Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6:163–7.
Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7:607–10.
Voogd J. Comparative aspects of the structure and fibre connexions of the mammalian cerebellum. Prog Brain Res. 1967;25:94–134.
Hawkes R, Gravel C. The modular cerebellum. Prog Neurobiol. 1991;36:309–27.
Hawkes R. An anatomical model of cerebellar modules. Prog Brain Res. 1997;114:39–52.
Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–81.
Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496:303–13.
Marzban H, Kim CT, Doorn D, Chung SH, Hawkes R. A novel transverse expression domain in the mouse cerebellum revealed by a neurofilament-associated antigen. Neuroscience. 2008;153:1190–201.
Sawada K, Fukui Y, Hawkes R. Spatial distribution of corticotropin-releasing factor immunopositive climbing fibers in the mouse cerebellum: analysis by whole mount immunohistochemistry. Brain Res. 2008;1222:106–17.
Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol. 1999;412:95–111.
Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of Purkinje cells in the adult mouse cerebellar cortex. J Comp Neurol. 2000;416:383–97.
Akintunde A, Eisenman LM. External cuneocerebellar projection and Purkinje cell zebrin II bands: a direct comparison of parasagittal banding in the mouse cerebellum. J Chem Neuroanat. 1994;7:75–86.
Ji Z, Hawkes R. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience. 1994;61:935–54.
Sugihara I. Organization and remodeling of the olivocerebellar climbing fiber projection. Cerebellum. 2006;5:15–22.
Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci: Off J Soc Neurosci. 2004;24:8771–85.
Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol. 2007;500:1076–92.
Chockkan V, Hawkes R. Functional and antigenic maps in the rat cerebellum: zebrin compartmentation and vibrissal receptive fields in lobule IXa. J Comp Neurol. 1994;345:33–45.
Chen G, Hanson CL, Ebner TJ. Functional parasagittal compartments in the rat cerebellar cortex: an in vivo optical imaging study using neutral red. J Neurophysiol. 1996;76:4169–74.
Hallem JS, Thompson JH, Gundappa-Sulur G, Hawkes R, Bjaalie JG, Bower JM. Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIA of the rat. Neuroscience. 1999;93:1083–94.
Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–9.
De Camilli P, Miller PE, Levitt P, Walter U, Greengard P. Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience. 1984;11:761–817.
Marzban H, Khanzada U, Shabir S, Hawkes R, Langnaese K, Smalla KH, et al. Expression of the immunoglobulin superfamily neuroplastin adhesion molecules in adult and developing mouse cerebellum and their localisation to parasagittal stripes. J Comp Neurol. 2003;462:286–301.
Hawkes R, Herrup K. Aldolase C/zebrin II and the regionalization of the cerebellum. J Mol Neurosci: MN. 1995;6:147–58.
Furuya S, Makino A, Hirabayashi Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res Brain Res Protocols. 1998;3:192–8.
Tabata T, Sawada S, Araki K, Bono Y, Furuya S, Kano M. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J Neurosci Methods. 2000;104:45–53.
Marzban H, Hawkes R. Fibroblast growth factor promotes the development of deep cerebellar nuclear neurons in dissociated mouse cerebellar cultures. Brain Res. 2007;1141:25–36.
Jande SS, Tolnai S, Lawson DE. Immunohistochemical localization of vitamin D-dependent calcium-binding protein in duodenum, kidney, uterus and cerebellum of chickens. Histochemistry. 1981;71:99–116.
Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J Neurosci. 2010;30:10015–24.
Reeber SL, White JJ, George-Jones NA, Sillitoe RV. Architecture and development of olivocerebellar circuit topography. Front Neural Circ. 2012;6:115.
Eisenman LM, Hawkes R. Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J Comp Neurol. 1993;335:586–605.
Marzban H, Zahedi S, Sanchez M, Hawkes R. Antigenic compartmentation of the cerebellar cortex in the Syrian hamster Mesocricetus auratus. Brain Res. 2003;974:176–83.
Hawkes R. Antigenic markers of cerebellar modules in the adult mouse. Biochem Soc Trans. 1992;20:391–5.
Eisenman LM, Hawkes R. 5′-Nucleotidase and the mabQ113 antigen share a common distribution in the cerebellar cortex of the mouse. Neuroscience. 1989;31:231–5.
Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10:422–34.
Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408.
Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–91.
Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain–hindbrain malformations. Brain: J Neurol. 2009;132:3199–230.
Melquist S, Craig DW, Huentelman MJ, Crook R, Pearson JV, Baker M, et al. Identification of a novel risk locus for progressive supranuclear palsy by a pooled genomewide scan of 500,288 single-nucleotide polymorphisms. Am J Hum Genet. 2007;80:769–78.
Metcalf DJ, Calvi AA, Seaman M, Mitchison HM, Cutler DF. Loss of the Batten disease gene CLN3 prevents exit from the TGN of the mannose 6-phosphate receptor. Traffic. 2008;9:1905–14.
Saftig P, Hartmann D, Lullmann-Rauch R, Wolff J, Evers M, Koster A, et al. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J Biol Chem. 1997;272:18628–35.
Tanaka Y, Himeno M, Kato K. Release of acid phosphatase from lysosomal membranes by cathepsin D. J Biochem. 1990;108:287–91.
Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17.
Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–45.
Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, et al. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–29.
Chung SH, Kim CT, Hawkes R. Compartmentation of GABA B receptor2 expression in the mouse cerebellar cortex. Cerebellum. 2008;7:295–303.
Terada N, Banno Y, Ohno N, Fujii Y, Murate T, Sarna JR, et al. Compartmentation of the mouse cerebellar cortex by sphingosine kinase. J Comp Neurol. 2004;469:119–27.
Marzban H, Sillitoe RV, Hoy M, Chung SH, Rafuse VF, Hawkes R. Abnormal HNK-1 expression in the cerebellum of an N-CAM null mouse. J Neurocytol. 2004;33:117–30.
Consalez GG, Hawkes R. The compartmental restriction of cerebellar interneurons. Front Neural Circ. 2012;6:123.
Sillitoe RV, Marzban H, Larouche M, Zahedi S, Affanni J, Hawkes R. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog Brain Res. 2005;148:283–97.
Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70:473–507.
McGovern MM, Schuchman EH. Acid sphingomyelinase deficiency. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. Gene reviews. Seattle: University of Washington; 1993.
Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann–Pick type C disease. J Comp Neurol. 2003;456:279–91.
Amritraj A, Peake K, Kodam A, Salio C, Merighi A, Vance JE, et al. Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann–Pick type C1-deficient mice. Am J Pathol. 2009;175:2540–56.
Butler D, Hwang J, Estick C, Nishiyama A, Kumar SS, Baveghems C, et al. Protective effects of positive lysosomal modulation in Alzheimer’s disease transgenic mouse models. PLoS One. 2011;6:e20501.
Liang Q, Ouyang X, Schneider L, Zhang J. Reduction of mutant huntingtin accumulation and toxicity by lysosomal cathepsins D and B in neurons. Mol Neurodegener. 2011;6:37.
Stromme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal–lysosomal dysfunction. Brain: J Neurol. 2011;134:3369–83.
Sachs AJ, David SA, Haider NB, Nystuen AM. Patterned neuroprotection in the Inpp4a(wbl) mutant mouse cerebellum correlates with the expression of Eaat4. PLoS One. 2009;4:e8270.
Acknowledgments
We thank Song Ren for technical assistance and Matt Hamaberg from the Genetic Model Center for his help in setting up and maintaining our mouse colony. We would also like to thank the Department Core Facility Center for the use of the equipment and Maike Bossert for her assistance. These studies were supported by grants from the Manitoba Health Research Council (HM) and the Manitoba Medical Service Foundation (HM).
Conflict of Interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bailey, K., Rahimi Balaei, M., Mehdizadeh, M. et al. Spatial and Temporal Expression of Lysosomal Acid Phosphatase 2 (ACP2) Reveals Dynamic Patterning of the Mouse Cerebellar Cortex. Cerebellum 12, 870–881 (2013). https://doi.org/10.1007/s12311-013-0502-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0502-y