Abstract
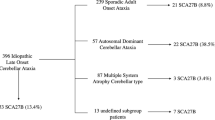
The autosomal dominant cerebellar ataxias (ADCAs) are a genetically heterogeneous group of disorders. Clinical classification of the ADCAs into three types has facilitated defining phenotypes and in turn, linkage analysis, which has led to the discovery of 30 loci and 16 genes. The type III ADCAs are ‘pure’ spinocerebellar ataxias (SCA), those that appear to elude neurological features outside of the cerebellum. At present 3 ADCA type III SCA genes have been published, SCA5, SCA6, and SCA14, these three genes appear to have various roles suggesting involvement in both different and possibly overlapping neurodegenerative pathways. The known ADCAIII genes are thought to have such roles as involvement in signal transduction, cell proliferation, synaptic transmission, and channel regulation. Here we update readers on the current progress on SCA11 and the identification of the disease gene. We discuss the clinical, genetic, and pathological details of SCA11 – a locus at chromosome 15q14-q21.3 in a Caucasian family of British ancestry. We also discuss the refining of this region, and methods used to prioritize the screening of the over 130 candidate genes in this genomic region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Harding AE. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias: A study of 11 families, including descendants of the Drew family of Walworth. Brain. 1982;105:1–28.
Zhuchenko O, Bailey J, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the a1A-voltage dependent calcium channel. Nature Genetics. 1997;15:6–9.
Chen D, Brkanac Z, et al. Missense mutations in the regulatory domain of PKC-gamma: A new mechanism for dominant nonepisodic cerebellar ataxia. Am J Human Genetics. 2003;72:839–49.
Ikeda Y, Dick K, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nature Genetics. 2006;38:184–90.
Diriong S, Lory P, et al. Chromosomal localization of the human genes for alpha-1A, alpha-1B, and alpha-1E voltagedependent Ca(2+) channel subunits. Genomics. 1995;30:605–09.
Burns D, Bell R. Protein kinase C contains two phorbol ester binding domains. J Biolog Chem. 1991;266:18330–8.
Ishikawa K, Toru S, et al. An autosomal dominant cerebellar ataxia linked to chromosome 16q22.1 is associated with a single-nucleotide substitution in the 5′ untranslated region of the gene encoding a protein with spectrin repeat and Rho guanine-nucleotide exhange-factor domains. Am J Human Genetics. 2005;77:280–96.
Flanigan K, Garnder K, et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): Clinical description and genetic localization to chromosome 16q22.1. Am J Human Genetics. 1996;59:392–9.
Worth P, Giunti P, et al. Autosomal dominant cerebellar ataxia type III: Linkage in a large British family to a 7.6cM region on chromosome 15q14-21.3. Am J Human Genetics. 1999;65:420–6.
Miyoshi Y, Yamada T, et al. A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1-24.1. Neurology. 2001;57:96–100.
Knight M, Kennerson M, et al. Spinocerebellar ataxia type 15 (SCA15) maps to 3p24.2-4pter: exclusion of the ITPR1 gene, the human orthologue of an ataxic mouse mutant. Neurobiolog Disorders. 2003;13:147–57.
Yu G-Y, Howell M, et al. Spinocerebellar ataxia type 26 maps to chromosome 19p13.3 adjacent to SCA6. Ann Neurol. 2005;57:349–54.
Ott J. Computer-simulation methods in human linkage analysis. Proc Natl Acad Sci. 1989;86:4175–8.
Lathrop G, Lalouel J. Easy calculations of LOD scores and genetic risks on small computers. Am J Human Genetics. 1984;36:460–5.
Sisodia S. Nuclear inclusions in glutamine repeat disorders: Are they pernicious, coincidental, or beneficial? Cell. 1998;95:1–4.
Margolis R, McInnis M, et al. Trinucleotide repeat expansion and neuropsychiatric disease. Arch Gen Psychiatry. 1999;56:1019–31.
Yuan Q, Schalling M. Detection and isolation of trinucleotide repeat expansions using the RED method. Meth Mol Biol. 2004;277:47–59.
Breschel T, McInnis M, et al. A novel, heritable, expanding CTG repeat in an intron of the SEF2-1 gene on chromosome 18q21.1. Human molecular genetics. 1997;6:1855–63.
Nakamoto M, Takebayashi H, et al. A CAG/CTG expansion in the normal population. Nature Genetics. 1997;17:385–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, J., Wood, N., Giunti, P. et al. Clinical and genetic analysis of spinocerebellar ataxia type 11. Cerebellum 7, 159–164 (2008). https://doi.org/10.1007/s12311-008-0022-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-008-0022-3
Key words
Profiles
- Henry Houlden View author profile