Abstract
The 2008 ICSH guideline was proposed to bring uniformity in bone marrow reporting among pathologists worldwide. The objective was to study the usefulness of on-site procedural information in routine marrow reporting. The nature of marrow aspirate, gross appearance, and core length of iliac crest trephine biopsies from 174 consecutive subjects (109 males, 65 females, mean age 47.5 ± 19.2 years) were reviewed by a pathologist blinded to the final diagnosis. The nature of aspirate had a significant correlation with a positive diagnostic yield among 149/176 (84.6%) cases (P = 0.03). Among cytopenic subjects, megaloblastic anemias (MA) (N = 27) and aplastic anemias (AA) (N = 14) had richly particulate and greasy/fat rich aspirates, respectively (P < 0.001). Eighty-six of 104 (82.7%) normal vs. 61/70 (87.1%) abnormal cores (for age) yielded a diagnosis (P = 0.43). Seventeen (63%) of the MA had a deep red trephine core (P = 0.18); and 12/14 (84.5%) of the AA had yellowish trephine core (P = 0.008). Though acute leukemias (N = 38) yielded variable aspirate materials (P = 0.34), they were more likely to have a pale, discolored trephine core (30/38 (79%) vs. 8/38 (21%), P < 0.001). A core length of ≥ 15 mm (N = 155) had higher diagnostic yield compared to < 15 mm (N = 19) (P = 0.05). Careful documentation of on-site marrow procedure information is necessary; and this may provide valuable clues for the morphological diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone marrow (BM) examination is a simple, cost-effective, and essential diagnostic tool for laboratory evaluation of unexplained peripheral blood cytopenia (s) and/or cytoses; investigation of abnormal peripheral blood smear (PBS) findings; diagnosis, follow-up, and staging of several hematolymphoid malignancies like acute leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic and/or myeloproliferative neoplasms, plasma cell dyscrasias; suspected marrow metastasis from solid malignancies; fever of unknown origin (FUO); storage disorders; for exclusion of hematological disease, if any, in potential donors of allogenic hematopoietic stem cell transplantation; and last but not the least for submission of marrow aspirate for several ancillary studies useful for patient diagnoses [1,2,3]. In order to maintain uniformity in BM reporting among hematopathologists worldwide, the International Council for Standardisation in Hematology (ICSH) formulated a guideline for standardization of bone marrow processing and reporting in the year 2008 which is currently in wide usage worldwide [4]. This guideline takes into consideration the relevant preanalytical variables such as clinical examination findings, presence or absence of organomegaly and/or lymphadenopathy, complete blood count, and peripheral smear examination findings which need to be integrated into BM aspirate (BMA) and trephine touch imprint cytology along with trephine biopsy (BMBx) histomorphology, supplemented with results of pertinent ancillary investigations (wherever necessary) for an accurate diagnosis [5]. One important preanalytical information, although incorporated in the 2008 ICSH guidelines but rarely documented in the BM requisition form, is the on-site procedure information related to nature of aspirated material, trephine core length (CL), and its gross appearance. However, there has been a paucity of literature surrounding the gross appearance of BM trephine core biopsy and its diagnostic utility in routine hematopathology practice. In this manuscript, we aim to present our data on 176 consecutive cases with special references to nature of BM aspirate and gross appearances of BM trephine biopsy with their impact on diagnostic outcome.
Materials and methods
The archival BM records of all the subjects who underwent BM procedure over a period of 2 years (January 2014 to December 2015) at the Department of Pathology at Pondicherry Institute of Medical Sciences, Pondicherry, India, were retrospectively reviewed. The Institutional Ethics Committee approved the retrospective record review and this study was conducted as per the Declaration of Helsinki. After obtaining the patient consent, BM procedure was undertaken by a consultant pathologist (SP) trained in BM procedure or a resident under his supervision from posterior superior iliac spine in lateral position under 2% xylocaine infiltration anaesthesia under strict aseptic precautions following a double needle technique (DNT) [6, 7]. In all cases, aspirate was taken first using Salah bone marrow aspiration needle prior to obtaining BMBx. Trephine biopsy was performed using Jamshidi™ biopsy needle (11G × 9 cm for adults, 13G × 9 cm for pediatric subjects less than 10 years of age; CareFusion, USA) through the same skin incision site but at a different point on the bone 0.5 to 1 cm apart; and following a different angle laterally (so-called lateral approach) [8]. In total, 1 to 2 ml BMA was slowly pulled into a 5-ml syringe containing 0.5 ml of 1% dipotassium EDTA, quickly mixed, and then poured drop-wise onto a slanted clean glass slide allowing the excess blood to be drained off the slide into the petri dish. Then, marrow fragments/particles were carefully taken out with the edge of another clean glass slide onto 5 to 6 slides to prepare “particle crush/squash” smears (Supplement 1, S1). As a routine practice at our center, the smears were prepared by the consultant pathologist or the pathology residents who described the nature of aspirate material as follows: (i) aparticulate/hemodiluted, (ii) scant or tiny particulate, (iii) greasy (fat rich, oily), (iv) particulate, (iv) richly particulate, (v) clotted, (vi) dry tap (Fig. 1). Similarly, after extraction from the biopsy needle, the trephine core was gently rolled on a filter paper; and then the gross appearance and prefixation core length (pre-CL) (including the periosteum, cortex, and/or cartilage) were noted down carefully. The following descriptors were used to describe the gross appearance of trephine core such as normal or abnormal for age; the latter further described as follows: (i) deep red/cherry red, (ii) fatty/yellowish/greasy, (iii) pale (discolored), (iv) soft/slimy. This was followed by preparation of minimum six “serial touch imprint” smears by gently touching a clean glass slide over it. Representative smears were stained with May-Grünwald-Giemsa (MGG) for cytomorphological assessment.
The trephine biopsy was fixed in 10% neutral buffered formalin for 8 to 10 h followed by slow decalcification using formol-citrate solution for 12 to 24 h before paraffin embedding. Two 4-µm-thick trephine sections were stained with haematoxylin eosin (H&E); and one each for per iodic acid Schiff (PAS) and Gomori silver reticulin staining for routine histomorphological evaluation. The following parameters were noted: number of evaluable intertrabecular marrow spaces (EIMS); age wise marrow cellularity; architectural distribution and morphology of trilineage hematopoiesis; grade of reticulin condensation on a 0 to 3 scale as per European consensus on the grading of bone marrow fibrosis [9]; stromal alteration; and presence or absence of any pathologic abnormality to reach a comprehensive morphologic diagnosis. The microscopic evaluation of BMA and BMBx was performed in accordance with 2008 International Council of Standardization in Hematology guidelines [5]. In all cases, the operator who performed and documented the aspirate and trephine core findings was blinded to the underlying diagnoses.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Science (SPSS, version 20.0) for Microsoft Windows. Descriptive statistics were presented as numbers and percentages for categorical variables; mean and SD for continuous variables. Chi-square test/Fisher’s exact test was used for qualitative data. The data was checked for normality and, thereafter, parametric/nonparametric tests were performed. Kruskal–Wallis test and Mann–Whitney test were used for nonnormally distributed continuous data. The One-Sample Proportion test was used to assess trephine core appearance. A two-sided P value < 0.05 was considered statistically significant.
Results
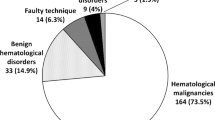
One hundred and seventy-six consecutive subjects underwent simultaneous BMA and BMBx procedure which included 111 males (63.1%) and 65 females (36.9%) with mean age of 47.5 ± 19.2 years (range 2 months to 85 years); and only 5 cases (2.8%) were under 14 years of age (Table 1). The four most common clinical indications were hematological malignancies (N = 74, 42%), fever of unknown origin (FUO) (N = 36, 35%), cytopenia(s) (N = 35, 35%), and staging for non-Hodgkin lymphoma (N = 20, 11%). Similarly, the four most common diagnoses offered by pathologists after BM examination were hematological malignancies (N = 66, 37%) (acute leukemias (N = 38), myelomas (N = 15), myeloproliferative neoplasm (N = 6), myelodysplastic syndrome (N = 4), chronic lymphoproliferative neoplasm (N = 3)); benign/nonspecific reactive change (N = 52, 30%); megaloblastic anemia (N = 27, 15%); and aplastic/hypoplastic marrow (N = 17, 10%).
In one hundred and thirty-four (76%) cases, the procedure yielded an “easy” aspirate; and it was reported as “difficult” in the remaining 42 subjects (24%). The aspirate samples were particulate in 88 (50%); richly particulate in 21 (12%); scant/tiny particulate with or without hemodilution in 37 (21%); aparticulate and hemodiluted in 14 (8%); greasy or fat like in 14 (8%), aparticulate and hemodiluted in 7 (4%), clotted in six (3%), and dry tap in remaining three (2%) cases. One hundred eleven among 134 (82.8%) cases with easy aspirate and thirty-eight of forty-two (90.5%) with difficult aspirate yielded a morphological diagnosis (P = 0.23). Similarly, eighty-six of 104 (82.7%) normal vs. 61/70 (87.1%) abnormal cores (for age) yielded a diagnosis (P = 0.43). Among 149 cases with a morphological diagnosis, 66 (44.3%) had particulate aspirate, 21 (14.1%) had richly particulate aspirate, 34 (22.8%) yielded scant particles, seven (4.7%) had greasy (fat rich) aspirate, twelve (8.1%) had aparticulate aspirate, three (2%) yielded dry tap; and the remaining six (4%) had clotted sample during the procedure. Similarly, among twenty-seven cases with no diagnosis offered on BMA examination, twenty-two (81.5%), three (11.1%), and two (7.4%) had particulate, scant particulate, and aparticulate aspirate, respectively (P = 0.03) (Table 2).
The association between the various morphological diagnoses with their nature of aspirate and corresponding trephine gross appearances is presented in Table 3. Megaloblastic anemia cases (N = 27) were more likely to have richly particulate than particulate aspirate smears (21/21 (100%) vs. 6/66 (9.0%), respectively, P < 0.001) and a deep red to cherry red trephine core than normal for age (17/27 (63%) vs. 10/27 (37%), respectively, P = 0.178). Aplastic/hypoplastic anemias (N = 14) were more likely to have greasy (fat rich) (7/7, 100%), aparticulate (5/12, 41.6%), and scant (2/34, 5.8%) particulate aspirate; and 12/14 (85.8%) of such marrow trephine cores were grossly abnormal (yellowish) (P < 0.001). Acute leukemias (N = 38) did not show any statistically significant association (P = 0.34) with aspirate quality; although a higher proportion of such cases were pale and discolored than normal cores (30 (79%) vs. 8 (21%), respectively, P < 0.001). Myelomas (N = 15) yielded particulate aspirate smears more often than scant (P = 0.21); and a very high proportion (14/15, 93.3%) had a soft and slimy trephine core on close examination (P = 0.001). The median age at myeloma diagnosis was 67 years (range 47 to 70 years). The only case which had a normal gross appearance had focal nodular aggregate of differentiated (non-blastic) myeloma cells which constituted 30% of marrow nucleated cells. On the other hand, cases with a “soft and slimy” trephine core (N = 14) had higher tumor burden (median 60%, range 40 to 90%) with interstitial to diffuse pattern of marrow involvement by tumor cells. Besides this, four of the latter had a plasmablastic morphology. Four of six (66.7%) CMPN (one primary myelofibrosis, three chronic myeloid leukemia) yielded hard, pale, discolored trephine cores representative of thickened and sclerotic trabeculae; 3/3 (100%) with gelatinous marrow transformation yielded soft, mucoid-like biopsy, whereas there was no statistically significant association with the gross appearance of trephine core among reactive/nonspecific category (P > 0.05) (Fig. 2a-f).
Abnormal trephine cores with their corresponding morphological diagnoses. a1–a3 Bone marrow morphology from a 45-year-old male with pancytopenia secondary to megaloblastic anemia. Note the cellular aspirate morphology depicting megaloblasts with opened up chromatin (a1), deep red trephine core (a2), and representative trephine histomorphology (a3) demonstrating sheets of erythroid precursors with peripherally marginated elongated nucleoli abutting the nuclear membrane so characteristic of megaloblasts in megaloblastic anemia. Also note one megakaryocyte (below) with nuclear lobe separation (a1 May-Grünwald-Giemsa, × 400, a3 hematoxylin eosin, × 400). b A hard, pale, discolored trephine core and the corresponding photomicrograph of a case from chronic phase chronic myeloid leukemia (CML) demonstrating a “pink tissue section” with 100% cellularity, myeloid hyperplasia, abnormal (dwarf) megakaryocytes, and markedly suppressed erythroid islands (hematoxylin eosin, × 200). c A grossly abnormal (yellowish, greasy) trephine core from a 37-year-old male with severe pancytopenia; and the corresponding photomicrographs demonstrating marked hypocellularity, suppressed trilineage hematopoiesis, and near total replacement by fat diagnostic of aplastic anemia (hematoxylin eosin, × 200). d A hard, pale, discolored linear core of trephine biopsy from a 65-year-old male with splenomegaly and pancytopenia demonstrating marrow hypocellularity, osteomyelosclerosis, thickened irregular bony trabeculae, and dilated sinusoids consistent with primary myelofibrosis (hematoxylin eosin, × 200). e A grossly abnormal linear core of trephine biopsy from a 24-year-old male with prolonged fever and severe weight loss secondary to pre-B cell acute lymphoblastic lymphoma (diagnosed from other side) demonstrating numerous tiny cavitary areas containing myxoid appearing substance. Note the aspirated scant, fibrillar, myxoid-like stromal matrix (above, right) (May-Grünwald-Giemsa, × 400) and corresponding trephine section showing gelatinous marrow transformation (hematoxylin eosin, × 200). f Bone marrow morphology from a case of multiple myeloma in a 47-year-old female. Bone marrow aspirate smears from the case showing myeloma cells with a flame cell morphology (f1, May-Grünwald-Giemsa, × 400). Note the abnormal linear core of trephine biopsy with patchy lytic areas (black arrow head) (f2); and the representative trephine histology demonstrating sheets of myeloma cells with increased osteoclastic activity (top left) (f3, hematoxylin eosin, × 400). The myeloma cells constituted 80% of marrow nuclear differential count
One hundred and fifty-five subjects (89%) had CL of ≥ 15 mm whereas the remaining nineteen (11%) had CL of < 15 mm. The mean CL was 23.63 ± 9.26 mm (range 9 to 50 mm); and CLs obtained from male and female subjects were comparable (24.71 ± 9.52 mm vs. 21.83 ± 8.57 mm, respectively, P > 0.05). Increasing CL was not associated with a diagnostic yield (P = 0.57) (Table 2).
Discussion
This retrospective review analyzed the trend in bone marrow sampling at a tertiary academic centre over a period of 2 years. As a part of quality assurance program and residential training, there was a shift in trend of BM procedure from being done by clinicians towards the pathologists; and unilateral marrow sampling was performed exclusively in all cases. In our center, benign marrow disorders outnumbered the hematological and non-hematological solid malignancies; and pediatric marrows were rarely performed. We obtained a satisfactory CL (≥ 15 mm) in a higher proportion (155/174 (89.1%)) of our subjects as per the recommended guideline [10,11,12]; though increasing the core length was not associated with improved diagnostic yield.
In slight deviation from the 2008 ICSH guidelines, we did a minor modification in aspirate sampling and smearing technique by collecting the BMA into EDTA containing syringe to avoid hemodilution and clotting. This technique was useful in preparing crush smears devoid of background blood elements and enhanced the quality of cytomorphological details in MGG staining. Moreover, the conditions which were expected to have a predilection for quicker clotting such as acute leukemia(s), especially APML, hypoplastic/aplastic anemias, HLH, etc., were better picked up by this sampling technique.
Among cytopenic subjects, megaloblastic anemias yielded richly particulate smears with very high cellularity. On the other hand, a greasy, lipoma-like aspirate with hypocellular (fat rich) smears was almost always diagnostic of marrow hypoplasia that correlated with trephine histology. Acute leukemias did not correlate with aspirate material, though cases with dry tap were better picked up in imprint cytosmears in the form of clusters and/or nodular aggregates of blasts. The architectural patterns such as clustering and nodular aggregates of plasma cells, and epithelioid cells were better appreciated on imprint smears than aspirates. The EDTA collection technique also enabled us to prepare “particle clot” tissue sections in myeloma subjects where getting an adequate, unbroken trephine core was a challenge due to the friable nature of bones.
We used DNT as well as a different angle to obtain the BMBx to prevent the “same needle track” core damage due to background hemorrhage. Also, we meticulously documented the macroscopic appearance after soaking off the extra blood from the core biopsy. Considering the age wise physiologic variation in hematopoietic marrow (red marrow), it is expected that proportion of red marrow decreases with age with corresponding increasing in fat cells (yellow marrow). This forms the basis of nearly 100% marrow hematopoietic cellularity among newborns and children to roughly equal proportion (50:50) of hematopoietic and fat elements in middle-aged individuals to greasy (fat rich) in the elderly population. Therefore, any variation in this physiology would be reflected on the alteration of the gross appearance of trephine core. In accordance with this, all histologically proven hypoplastic/aplastic marrows were grossly abnormal (yellowish) to the naked eyes. The myelomatous disease process weakens the bone making it more friable and prone for microfracture and thinned out trabeculae; and the cores were slimy or gelly-like making imprint smearing difficult in the latter. This was further corroborated by the increased tumor burden (advanced histologic stage) in 14/15 myeloma cases in our series which showed an abnormal gross morphology in comparison to the only case with a normal gross morphology as the disease process was patchy (focal nodular) in the latter with a lesser tumor burden. On the other hand, megaloblastic cores were deep red to cherry red in appearance due to erythroid-rich florid trilineage hyperplasia with reversal of myeloid to erythroid ratio. Similarly, both acute leukemias and particularly CML cores were grossly discolored to pale red in appearance due to suppressed trilineage hematopoiesis by the leukemic blasts in the former; and erythropenic florid myelo-megakaryocytic hyperplasia (M: E ranging from 10 to 15: 1) with or without associated myelofibrosis, osteosclerosis in the latter (Fig. 2b). The hard, pale, whitish appearing trephine core obtained from one of our cases of primary myelofibrosis demonstrated extensive osteomyelosclerosis, marrow hypocellularity, and irregularly thickened bony trabeculae (Fig. 2d). Consequently, the trephine tissue sections from acute leukemias and megaloblastic anemias appeared “purple” in hematoxylin eosin staining due to packed marrow-like pattern secondary to sheets of blasts in the former and florid eythroid hyperplasia in the latter. Similarly, all CML cores yielded “pink” tissue sections due to florid myeloid and/or megakaryocytic hyperplasia.
There has been a surge of data highlighting the impact of procedure techniques and type of needle used (single bevelled vs. triple bevelled; manual vs. powered devices, etc.) on the quality and adequacy of the marrow specimen obtained [6, 7, 13,14,15]. Some centers across the USA like Washington University School of Medicine do in fact practise collection of marrow aspirate into EDTA tube as well as performing a gross inspection of trephine cores by their operators (nurses) before submitting to the hematopathology department [13]. They reported that triple bevelled biopsy needles procure better length but microscopically evaluated inferior quality of trephine cores and more hemodiluted aspirate materials when compared with the same obtained by single bevelled biopsy needle. However, no correlation of gross findings was reported in that study [13]. We do also suggest that using a single insertion site single needle technique may have a more hemorrhagic (reddish) trephine core compared to double needle technique which may cause gross interpretation tricky.
The major strength of our study is that of meticulously describing the gross (macroscopic) appearance of trephine core biopsy from a sizable number of cases by a pathologist who was blinded to the underlying diagnoses. To the best of our knowledge, correlation between gross and microscopy in bone marrow trephine pathology has not been previously described in the literature. Using simple descriptors such as “normal” or “abnormal” for age was easily reproducible among operators. The retrospective nature of the review, possible operator bias, and non-inclusion of potential confounding factors could be considered limitations of our study. To conclude, abnormal appearance of trephine core may provide valuable clue for specific underlying bone marrow disease. Careful documentation of such on-site procedural information should be a part of mandatory preanalytical checklist as suggested by ICSH guidelines.
References
Le Clef Q, Mentera T, Tzankov A (2019) Our approach to bone marrow biopsies in cytopenia. Pathol Res Pract 215:152447
Tzankov A, Dirnhofer S, Beham-Schmid C (2012) Normal bone marrow and common reactive alterations. Pathologe 33:496–507
Fend F, Tzankov A, Bink K et al (2008) Modern techniques for the diagnostic evaluation of the trephine bone marrow biopsy: methodological aspects and applications. Prog Histochem Cytochem 42:203–252
Lee SH, Erber WN, Porwit A et al (2008) International Council for Standardization in Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol 30:349–364
Gilotra M, Gupta M, Singh S et al (2017) Comparison of bone marrow aspiration cytology with bone marrow trephine biopsy histopathology: an observational study. J Lab Physicians 9:182–189
Al-Ibraheemi A, Pham T, Chen L et al (2013) Comparison between 1-needle technique versus 2-needle technique for bone marrow aspiration and biopsy procedures. Arch Pathol Lab Med 137:974–978
Dayton VJ (2014) Quality and adequacy of bone marrow samples obtained by the 2-needle technique: the Minnesota experience. Arch Pathol Lab Med 138:860–862
Konda B, Pathak P, Edwin I et al (2014) Safe and successful bone marrow biopsy: an anatomical and CT-based cadaver study. Am J Hematol 89:943–946
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A (2005) European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90:1128–1132
Merzianu M, Groman A, Hutson A et al (2018) Trends in bone marrow sampling and core biopsy specimen adequacy in the united states and Canada: a multicenter study. Am J Clin Pathol 150:393–405
Goyal S, Singh UR, Rusia U (2014) Comparative evaluation of bone marrow aspirate with trephine biopsy in hematological disorders and determination of optimum trephine length in lymphoma infiltration. Mediterr J Hematol Infect Dis 6:e2014002
Arber DA, Orazi A, Hasserjian RP et al (2017) Introduction and overview of the classification of myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pilieri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R (eds) WHO classification of tumours of haematopoietic and lymphoid tissue. IARC, Lyon
Brestoff JR, Toland A, Afaneh K et al (2019) Bone marrow biopsy needle type affects core biopsy specimen length and quality and aspirate hemodilution. Am J Clin Pathol 151:185–193
Forwood KM, Lee E, Crispin PJ (2019) Comparison of the bone marrow trephine sample quality between OnControl drill system and the Jamshidi needle. Int J Lab Hematol 41:373–379
Odejide OO, Cronin AM, DeAngelo DJ et al (2013) Improving the quality of bone marrow assessment: impact of operator techniques and use of a specimen preparation checklist. Cancer 119(19):3472–3478
Author information
Authors and Affiliations
Contributions
SP designed the study, performed the bone marrow procedure, collected and interpreted the data, and wrote and edited the manuscript. KR did the statistical analysis of data. RGV and SP did the morphological evaluation of the marrows. AB, SM, and NI are clinicians who recruited the cases for bone marrow evaluation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
Sampling and smearing techniques: A; Particulate marrow fragments on a slanted glass slide, B; preparation of squash smears (JPG 89 kb)
Rights and permissions
About this article
Cite this article
Padhi, S., Ravichandran, K., Varghese, R.G. et al. Bone marrow aspiration and gross appearance of trephine biopsy in routine practice: a preliminary descriptive data on 176 consecutive cases from a single tertiary care center in South India. J Hematopathol 14, 117–124 (2021). https://doi.org/10.1007/s12308-021-00449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-021-00449-5