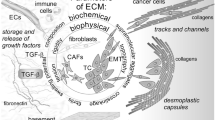
Abstract
The stroma is a considerable part of the tumor microenvironment. Because of its complexity, it can influence both cancer and immune cells in their behavior and cross-talk. Aside from soluble products released by non-cancer and cancer cells, extracellular matrix components have been increasingly recognized as more than just minor players in the constitution, development and regulation of the tumor microenvironment. The variations in the connective scaffold architecture, induced by transforming growth factor beta, lysyl oxidase and metalloproteinase activity, create different conditions of ECM density and stiffness. They exert broad effects on immune cells (e.g. physical barriers, modulation by release of stored TGF-β1), mesenchymal cells (transition to myofibroblasts), epithelial cells (epithelial-to-mesenchymal transition), cancer cells (progression to metastatic phenotype) and stem cells (activation of differentiation addressed by the microenvironment characteristics). Physiological mechanisms of the wound healing process, as well as mechanisms of fibrosis in some chronic pathologies, closely recall aspects of cancer deregulated biology. Their elucidation can provide a better understanding of tumor microenvironment immunobiology. In the following short review, we will focus on some aspects of the fibrous stroma to highlight its active participation in the tumor microenvironment constitution, tumor progression and the local immunological network.
Similar content being viewed by others
References
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Sautès-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, Dieu-Nosjean MC (2011) Tumor microenvironment is multifaceted. Cancer Metastasis Rev 30:13–25
Wu Y, Antony S, Meitzler JL, Doroshow JH (2013) Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 345:164–173
Taipale J, Miyazono K, Heldin CH, Keski-Oja J (1994) Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol 124:171–181
Unsöld C, Hyytiäinen M, Bruckner-Tuderman L, Keski-Oja J (2001) Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J Cell Sci 114:187–197
Valenick LV, Hsia HC, Schwarzbauer JE (2005) Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp Cell Res 309:48–55
Wipff PJ, Hinz B (2008) Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol 87:601–615
Monboisse JC, Oudart JB, Ramont L, Brassart-Pasco S, Maquart FX (2014) Matrikines from basement membrane collagens: A new anti-cancer strategy. Biochim Biophys Acta 1840:2589-2598
Egeblad M, Rasch MG, Weaver VM (2010) Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 22:697–706
Friedl P, Bröcker EB, Zänker KS (1998) Integrins, cell matrix interactions and cell migration strategies: fundamental differences in leukocytes and tumor cells. Cell Adhes Commun 6:225–236
Friedl P, Bröcker EB (2000) T cell migration in three-dimensional extracellular matrix: guidance by polarity and sensations. Dev Immunol 7:249–266
Miles FL, Sikes RA (2014) Insidious changes in stromal matrix fuel cancer progression. Mol Cancer Res 12:297–312
Peranzoni E, Rivas-Caicedo A, Bougherara H, Salmon H, Donnadieu E (2013) Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci 70:4431–4448
Dvorak HF (1986) Tumors: wounds that do not heal. N Engl J Med 315:1650–1659
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Cassetta L, Cassol E, Poli G (2011) Macrophage polarization in health and disease. Sci World J 11:2391–2402
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229:176–185
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14:986–995
Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW (2013) The role of immune-related myeloid cells in angiogenesis. Immunobiology 218:1370–1375
Pawelec G (2004) Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother 53:262–274
Croci DO, Zacarías Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG (2007) Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother 56:1687–1700
Weidenbusch M, Anders HJ (2012) Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun 4:463–477
Mantovani A (2012) MSCs, macrophages, and cancer: a dangerous ménage-à-trois. Cell Stem Cell 11:730–732
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A (2013) Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol 228:1404–1412
Li B, Wang JH (2011) Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 20:108–120
Polanska UM, Orimo A (2013) Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol 228:1651–1657
Byun JS, Gardner K (2013) Wounds that will not heal: pervasive cellular reprogramming in cancer. Am J Pathol 182:1055–1064
Liguori M, Solinas G, Germano G, Mantovani A, Allavena P (2011) Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers (Basel) 3:3740–3761
Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T (2008) Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol 83:1136–1144
Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, Voronov E (2013) The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol 190:3500–3509
Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39:1003–1018
Rider P, Carmi Y, Voronov E, Apte RN (2013) Interleukin-1α. Semin Immunol 25:430–438
Perrot CY, Javelaud D, Mauviel A (2013) Insights into the Transforming Growth Factor-β Signaling Pathway in Cutaneous Melanoma. Ann Dermatol 25:135–144
Jakowlew SB (2006) Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev 25:435–457
Petrella BL, Vincenti MP (2012) Interleukin-1β mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein β. Cancer Med 1:17–27
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26
Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K (2008) Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 111:4571–4579
Wong SF, Lai LC (2001) The role of TGFbeta in human cancers. Pathology 33:85–92
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S (2012) TGF-β - an excellent servant but a bad master. J Transl Med 10:183
Pickup M, Novitskiy S, Moses HL (2013) The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13(11):788–799
Kinashi H, Ito Y, Mizuno M, Suzuki Y, Terabayashi T, Nagura F, Hattori R, Matsukawa Y, Mizuno T, Noda Y, Nishimura H, Nishio R, Maruyama S, Imai E, Matsuo S, Takei Y (2013) TGF-β1 promotes lymphangiogenesis during peritoneal fibrosis. J Am Soc Nephrol 24:1627–1642
Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ (2008) TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295:H2113–H2127
Ji RC (2006) Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev 25:677–694
Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C (2007) Tumor evasion of the immune system by converting CD4 + CD25- T cells into CD4 + CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol 178:2883–2892
Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM, Huang RP (2014) Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 1845:182–201
Horiguchi M, Ota M, Rifkin DB (2012) Matrix control of transforming growth factor-β function. J Biochem 152:321–329
Taipale J, Saharinen J, Keski-Oja J (1998) Extracellular matrix-associated transforming growth factor-beta: role in cancer cell growth and invasion. Adv Cancer Res 75:87–134
Shahinian H, Loessner D, Biniossek ML, Kizhakkedathu JN, Clements JA, Magdolen V, Schilling O (2014) Secretome and degradome profiling shows that Kallikrein-related peptidases 4, 5, 6, and 7 induce TGFβ-1 signaling in ovarian cancer cells. Mol Oncol 8:68–82
Xiao Q, Ge G (2012) Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron 5:261–273
Kim YM, Kim EC, Kim Y (2011) The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep 38:145–149
Cano A, Santamaría PG, Moreno-Bueno G (2012) LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol 8:1095–1108
Zaffryar-Eilot S, Marshall D, Voloshin T, Bar-Zion A, Spangler R, Kessler O, Ghermazien H, Brekhman V, Suss-Toby E, Adam D, Shaked Y, Smith V, Neufeld G (2013) Lysyl oxidase-like-2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis 34:2370–2379
Barker HE, Bird D, Lang G, Erler JT (2013) Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res 11:1425–1436
Sion AM, Figg WD (2006) Lysyl oxidase (LOX) and hypoxia-induced metastases. Cancer Biol Ther 5:909–911
Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM, Moses HL (2013) Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res 73:5336–5346
Leask A, Hutchenreuther J (2014) Activation of latent TGFβ by α(v)β (1) integrin: of potential importance in myofibroblast activation in fibrosis. J Cell Commun Signal 8:171–172
Wipff PJ, Rifkin DB, Meister JJ, Hinz B (2007) Myofibroblast contraction activates latent TGF-β 1 from the extracellular matrix. J Cell Biol 179:1311–1323
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Calon A, Tauriello DV, Batlle E (2014) TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol 25:15–22
Desmoulière A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13:7–12
Montesano R, Orci L (1988) Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A 85:4894–4897
Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B (2011) The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21:2046–2054
Scharenberg MA, Pippenger BE, Sack R, Zingg D, Ferralli J, Schenk S, Martin I, Chiquet-Ehrismann R (2014) TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J Cell Sci 127:1079–1091
Vi L, de Lasa C, DiGuglielmo GM, Dagnino L (2011) Integrin-linked kinase is required for TGF-β1 induction of dermal myofibroblast differentiation. J Investig Dermatol 131:586–593
Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y (2012) Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47:340–348
Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281
Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP (2006) More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Investig 35:297–325
Chien AJ, Conrad WH, Moon RT (2009) A Wnt survival guide: from flies to human disease. J Investig Dermatol 129:1614–1627
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205
Chen D, Jarrell A, Guo C, Lang R, Atit R (2012) Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 139:1522–1533
O’Reilly S (2014) Innate immunity in systemic sclerosis pathogenesis. Clin Sci (Lond) 126:329–337
Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, Gottardi CJ, Atit R, Whitfield ML, Varga J (2012) Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum 64:2734–2745
Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, Sonnylal S, de Crombrugghe B, Taketo MM, Distler O, Schett G, Distler JH (2012) β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71:761–767
Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149:1192–1205
Kato S, Hayakawa Y, Sakurai H, Saiki I, Yokoyama S (2014) Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci 105:281–289
Díaz Prado SM, Medina Villaamil V, Aparicio Gallego G, Blanco Calvo M, López Cedrún JL, Sironvalle Soliva S, Valladares Ayerbes M, García Campelo R, Antón Aparicio LM (2009) Expression of Wnt gene family and frizzled receptors in head and neck squamous cell carcinomas. Virchows Arch 455:67–75
Qurrat-Ul-Ain, Seemab U, Nawaz S, Rashid S (2011) Integrative analyses of conserved WNT clusters and their co-operative behaviour in human breast cancer. Bioinformation 7:339–46
Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak MS, Wolsky R, Bentolila LA, Grant SG, Elashoff D, Lehr S, Latimer JJ, Bose S, Sattar H, Krum SA, Miranda-Carboni GA (2013) WNT10B/β-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med 5:264–279
Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C (2013) Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73:5657–5668
Kakudo N, Kushida S, Suzuki K, Ogura T, Notodihardjo PV, Hara T, Kusumoto K (2012) Effects of transforming growth factor-beta1 on cell motility, collagen gel contraction, myofibroblastic differentiation, and extracellular matrix expression of human adipose-derived stem cell. Hum Cell 25:87–95
Baarsma HA, Spanjer AI, Haitsma G, Engelbertink LH, Meurs H, Jonker MR, Timens W, Postma DS, Kerstjens HA, Gosens R (2011) Activation of WNT/β-catenin signaling in pulmonary fibroblasts by TGF-β1 is increased in chronic obstructive pulmonary disease. PLoS One 6:e25450
van Dekken H, Wink JC, Vissers KJ, Franken PF (2007) Ruud Schouten W, J Hop WC, Kuipers EJ, Fodde R, Janneke van der Woude C. Wnt pathway-related gene expression during malignant progression in ulcerative colitis. Acta Histochem 109:266–272
Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C (2011) Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol 179:2660–2673
Blaauboer ME, Emson CL, Verschuren L, van Erk M, Turner SM, Everts V, Hanemaaijer R, Stoop R (2013) Novel combination of collagen dynamics analysis and transcriptional profiling reveals fibrosis-relevant genes and pathways. Matrix Biol 32:424–431
Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26:730–738
Lui C, Lee K, Nelson CM (2012) Matrix compliance and RhoA direct the differentiation of mammary progenitor cells. Biomech Model Mechanobiol 11:1241–1297
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15:637–646
Acknowledgments
The author thanks the support of IAA500200917; CZ.1.05/2.1.00/03.0124 (Project ExAM); Anna Villa and Felice Rusconi Foundation, Varese (IT); UniCredit Bank, Prague (CZ); Manghi Group s.r.o., Prague (CZ); ARPA Foundation, Pisa (IT); RVO 61388971 and RVO 67985904 (CZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vannucci, L. Stroma as an Active Player in the Development of the Tumor Microenvironment. Cancer Microenvironment 8, 159–166 (2015). https://doi.org/10.1007/s12307-014-0150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-014-0150-x