Abstract
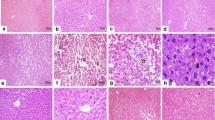
Caffeic acid is a well-known phenolic compound widely present in plant kingdom. The aim of this study was to investigate the possible protective effect of caffeic acid (CA) against oxytetracycline (OXT) induced hepatotoxicity in male Albino Wistar rats. A total of 30 rats weighing 150–170 g were randomly divided into five groups of six rats in each group. Oral administration of OXT (200 mg/kg body weight/day) for 15 days produced hepatic damage as manifested by a significant increase in serum hepatic markers namely aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), bilirubin and increased plasma and hepatic lipid peroxidation indices (TBARS and hydroperoxide). The present finding shows that the levels of enzymatic antioxidants namely superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) were significantly decreased in OXT intoxicated rats. Upon oral administration of caffeic acid (40 mg/kg body weight/day) there were decreased hepatic marker activities, bilirubin and lipid peroxidation and increased enzymatic antioxidants in OXT + Caffeic acid group compared to Normal + OXT group(P < 0.05). Our study suggests that caffeic acid has antioxidant property and hepatoprotective ability against OXT induced toxicity.
Similar content being viewed by others
References
Saraswat B, Viseu PKS, Patnaik GK, Dhawan BN. Protective effects of picroliv active constituent of picroorhiza kurrooa against oxytetracycline induced hepatic damage. Indian J Exp Biol. 1997;35:1302–5.
Gulcin I. Antioxidant activity of caffeic acid. Toxicology. 2006;217:213–20.
Kroon PA, Williamson G. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric. 1999;79:355–61.
Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–604.
Frank H, Thiel D, Macleod J. Mass spectroscopic detection of cross-linked fatty acids formed during radical induced lesion of lipid membranes. Biochem J. 1989;260:873–5.
Gebhardt R, Fausel M. Antioxidant and hepatoprotective effects of artichoke extract and constituents in cultured rat hepatocytes. Toxicology. 1997;11:669–72.
Joyeux M, Lobstein A, Anton R, Mortier F. Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflawones from ginkgo and some flavonoids. Planta Med. 1995;61:126–9.
Challis BC, Bartlett CD. Possible cocarcinogenic effects of coffee constituents. Nature. 1975;254:532–3.
Iwahashi H, Ishii T, Suguta R, Kido R. The effect of caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthranilic acid, ferric chloride and hydrogen peroxide. Arch Biochem Biophys. 1990;276:242–7.
Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28.
Sudina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21–4.
Rao MV, Paliyath G, Ormrod DP. Ultraviolet-band ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–36.
Meyer AS, Donovan JL, Pearson DA, Waterhouse AL, Frankel EN. Fruit hydroxycinnamic acids inhibit low density lipoprotein oxidation in vitro. J Agric Food Chem. 1998;46:1783–7.
Cartron E, Carbonneau MA, Fouret G, Descomps B, Leger CL. Specific antioxidant activity of caffeoyl derivatives and other natural phenolic compounds: LDL protection against oxidation and decrease in the proinflammatory lysophosphatidylcholine production. J Nat Prod. 2001;64:480–6.
Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50:2161–8.
Fraga CG, Leibovity BF, Topeel AL. Lipid peroxidation measured as TBARS in tissue slices-Characterization and comparison with homogenate and microsomes. Free Rad Biol Med. 1988;4:155–61.
Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–7.
Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of SOD. Indian J Biochem Biophys. 1984;21:130–2.
Sinha KA. Colorimetric assay of catalase. Ann Biochem. 1972;47:389–94.
Rotruck JT, Pope AL, Ganther HE, Swason AB. Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90.
Lowry OH, Rosebrough MH, Farr L, Randell RJ. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;93:265–75.
Duncan BD. Multiple range test for correlated and heteroscedastic means. Biometrics. 1957;13:359–64.
Janbaz K, Saeed S, Gilani A. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11:424–30.
Srinivasan M, Rukkumani R, Ram Sudheer A, Menon VP. Ferulic acid, a natural protector against carbon tetrachloride-induced toxicity. Fundam Clin Pharmacol. 2005; 19:491–6.
Zhao C, Shi Y, Lin W, Wang W, Jia Z, Yao S, Fan B, Zheng R. Fast repair of the radical cations of dCMP and poly C quercetin and rutin. Mutagenesis. 2001;16:271–5.
Gupta YK, Sharma M, Chaudhary G. Pyrogallol-induced hepatotoxicity in rats: a model to evaluate antioxidant hepatoprotective agents. Methods Find Exp Clin Pharmacol. 2002;24:497–500.
Maddrey WC. Drug induced hepatotoxicity. J Clin Gastroenterol. 2005;39:83–9.
Nardini M, Pisu P, Gentili V, Natella F, Di Felice M, Piccolella E, Scaccini C. Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress. Free Radic Biol Med. 1998;25:1098–105.
Jung UJ, Lee M-K, Park YB, Jeon S-M, Choi M-S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006;318:476–83.
Ozyurt B, Gulec M, Ozyurt H, Ekici F, Atis O, Akbas A. The effect of antioxidant caffeic acid phenethyl ester (CAPE) on some enzyme activities in cisplatin-induced neurotoxicity in rats. Eur J Gen Med. 2006;3:167–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayanthi, R., Subash, P. Antioxidant Effect of Caffeic Acid on Oxytetracycline Induced Lipid Peroxidation in Albino Rats. Ind J Clin Biochem 25, 371–375 (2010). https://doi.org/10.1007/s12291-010-0052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-010-0052-8