Abstract
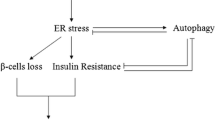
The endoplasmic reticulum (ER) is a cellular compartment responsible for multiple important cellular functions including the biosynthesis and folding of newly synthesized proteins destined for secretion, such as insulin. A myriad of pathological and physiological factors perturb ER function and cause dysregulation of ER homeostasis, leading to ER stress. Accumulating evidence suggests that ER stress plays a role in the pathogenesis of diabetes, contributing to pancreatic β-cell loss and insulin resistance. ER stress may also link obesity, inflammation and insulin resistance in type 2 diabetes. In this review, we address the transition from physiology to pathology, namely how and why the physiological UPR evolves to a proapoptotic ER stress response in diabetes and its complications. Special attention was given to elucidate how ER stress could explain some of the ‘clinical paradoxes’ such as secondary sulfonylurea failure, initial worsening of retinopathy during tight glycemic control, insulin resistance induced by protease inhibitors and other clinically relevant observations.
Similar content being viewed by others
References
Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J 2008;27:315–327.
Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 2005;569:29–63.
Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992;355:33–45.
Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 1999;13:1211–1233.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev Mol Cell Biol 2007;8:519–529.
Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005;74:739–789.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumour necrosis factor alpha: direct role in obesity linked insulin resistance. Science 1993;259:87–91.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 15:306(5695): 457–461.
Harding HP. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 2001;7:1153–1163.
Scheuner D. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 2001;7:1165–1176.
Scheuner D. Control of mRNA translation preserves endoplasmic reticulum function in β cells and maintains glucose homeostasis. Nature Med 2005;11:757–764.
Pirot P, Ortis F, Cnop M, Ma Y, Hendershot LM, Eizirik DL, et al. Transcriptional regulation of the endoplasmic reticulum stress gene chop in pancreatic insulin-producing cells. Diabetes 2007;56(4):1069–1077.
Harding HP, Ron D. Endoplasmic Reticulum Stress and the Development of Diabetes. Diabetes 2002;3:51.
Xu C, Bailly MB, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 2005;115(10):2656–2664.
Sundar RS, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Ind J Med Res 2007;125(3):411–424.
Deborah M, Muoio, Christopher B. Newgard Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature Reviews Mol Cell Biol 2008;9:193–205.
Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008;7(12):1013–1030.
Lin JH, Walter P, Benedict Y. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annual Review of Pathology: Mechanisms of Disease 2008;3:399–425.
Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29(1):42–61.
Boden G. Endoplasmic Reticulum Stress: Another Link between Obesity and Insulin Resistance/Inflammation? Diabetes 2009;58:518–519.
Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol 2009;9(6):763–770.
Hotamisligil GS. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010;140:900–917.
McAlpine CS, Bowes AJ, Werstuck GH. Diabetes, Hyperglycemia and Accelerated Atherosclerosis: Evidence Supporting a Role for Endoplasmic Reticulum (ER) Stress Signaling. Cardiovasc Hematol Disord Drug Targets 2010.
Srinivasan V, Tatu U, Mohan V, Balasubramanyam M. Molecular convergence of hexosamine biosynthetic pathway and ER stress leading to insulin resistance in L6 skeletal muscle cells. Mol Cell Biochem 2009;328(1–2):217–224.
Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type2diabetes mellitus progressive requirement for multiple therapies (UKPDS 49). JAMA 1999:2005–2012.
Iwakura T, Fujimoto S, Kagimoto S, Inada A, Kubota A, Someya Y, et al. Sustained enhancement of Ca2+ influx by glibenclamide induces apoptosis in RINm5F cells. Biochem Biophys Res Commun 2000;271:422–428.
Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 2005;54:452–461.
UK Prospective Diabetes Study (UKPDS) Group. Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443.
Inoguchi T, Umeda F, Kakimoto M, Sako Y, Ishii H, Noda K, et al. Chronic sulfonylurea treatment and hyperglycemia aggravate disproportionately elevated plasma proinsulin levels in patients with type 2 diabetes. Endocr J 2000;47:763–770.
Dworacka M, Abramczyk M, Winiarska H, Kuczynski S, Borowska M, Szczawinska K, et al. Disproportionately elevated proinsulin levels in type 2 diabetic patients treated with sulfonylurea. Int J Clin Pharmacol Ther 2006;44:14–21.
Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760.
Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet 1976;444–447.
Sargsyan E, Ortsater H, Thorn K, Bergsten P. Diazoxide-induced beta-cell rest reduces endoplasmic reticulum stress in lipotoxic betacells. J Endocrinol 2008;199(1):41–50.
Lamontagne J, Pepin E, Peyot ML, Joly E, Ruderman NB, Poitout V et al. Pioglitazone Acutely Reduces Insulin Secretion and Causes Metabolic Deceleration of the Pancreatic β-Cell at Submaximal Glucose Concentrations. Endocrinol 2009;150,8:3465–3474.
Aston-Mourney K, Proietto J, Morahan J, Andrikopoulos S. Too much of a good thing: why it is bad to stimulate the beta cell to secrete insulin. Diabetol 2008;51:540–545.
Qian L, Zhang S, Xu L, Peng Y. Endoplasmic reticulum stress in beta cells: latent mechanism of secondary sulfonylurea failure in type 2 diabetes? Med Hypotheses 2008;71(6):889–891.
Capeau J, Magre J, Lascols O, Caron M, Bereziat V, Vigouroux C, et al. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans 2005;3:1073–1077.
Lenhard JM, Furfine ES, Jain RJ, Ittoop O, Orband-Miller LA, Blanchard SG, et al. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. Antiviral Res 2000;47:121–129.
Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes 2003;53:1695–1700.
Barbaro G, Iacobellis G. Metabolic syndrome associated with HIV and highly active antiretroviral therapy. Curr Diab Rep 2009;9:37–42.
Parker RA, Flint OP, Mulvey R, Elosua C, Wang F, Fenderson W, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol 2005;67:1909–1919.
Djedaini M, Peraldi P, Drici MD, Darini C, Saint-Marc P, Dani C, et al. Lopinavir co-induces insulin resistance and ER stress in human adipocytes. Biochem Biophys Res Commun 2009;386(1):96–100.
Zha W, Liang G, Xiao J, Studer EJ, Hylemon PB, Pandak WM, et al. Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS One 2010;5(2):9069.
Ikesugi K, Mulhern ML, Madson CJ, Hosoya K, Terasaki T, Kador PF, et al. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr Eye Res 2006;31(11):947–953.
El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci 2003;44(7):3135–3143.
Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem 2004;279(15):14844–14852.
Oshitari T, Asaumi N, Watanabe M, Kumagai K, Mitamura Y. Severe macular edema induced by pioglitazone in a patient with diabetic retinopathy: a case study. Vasc Health Risk Manag 2008;4(5):1137–1140.
Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521–1527.
The Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmol 1995;102:647–661.
Dagogo-Jack S. Hypoglycemia in type 1 diabetes mellitus: pathophysiology and prevention. Treat Endocrinol 2004;3:91–103.
Heine RJ, Balkau B, Ceriello A, Del Prato S, Horton ES, Taskinen MR, et al. What does postprandial hyperglycaemia mean? Diabet Med 2004;21:208–213.
Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J 1985;290:811–815.
Funatsu H, Yamashita H, Ohashi Y, Ishigaki T. Effect of rapid glycemic control on progression of diabetic retinopathy. Jpn J Ophthalmol 1992;36:356–367.
Bijian K, Cybulsky AV. Stress proteins in glomerular epithelial cell injury. Contrib Nephrol 2005;148:8–20.
Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int 2010;77(3):187–193.
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, et al. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 2008;370(4):651–656.
Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, et al. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 2008;19(11):2225–2236.
Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for selfeating. Cell Death Differ 2005;12(Suppl 2):1542–1552.
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 2006;26, 9220–9231.
Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, et al. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 2006;3:211–222.
Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 2008;8:325–332.
Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 2008;8:318–324.
Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007;9:1102–1109.
Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, et al. Autophagymediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol Pharmacol 2009;76(3):596–603.
Ost A, Svensson K, Ruishalme I, Brannmark C, Franck N, Krook H, et al. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med 2010; Mar 26. [Epub ahead of print]
Wang HX, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci 1999;65:2663–2677.
Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, et al. Activation of AMP-activated Protein Kinase Inhibits oxidized Low Density Lipoprotein-triggered Endoplasmic Reticulum (ER) Stress in vivo. Diabetes 2010. [Epub ahead of print]
Kim DS, Jeong SK, Kim HR, Kim DS, Chae SW, Chae HJ. Metformin regulates palmitate-induced apoptosis and ER stress response in HepG2 liver cells. Immunopharmacol Immunotoxicol 2009 Dec 29. [Epub ahead of print]
Han KL, Choi SJ, Lee JY, Song J, Joe MK, Jung MH, et al. Therapeutic Potential of Peroxisome Proliferators-Activated Receptor-α/γ Dual Agonist With Alleviation of Endoplasmic Reticulum Stress for the Treatment of Diabetes. Diabetes 2008;57:737–745.
Yoshiuchi K, Kaneto H, Matsuoka TA, Kasami R, Kohno K, Iwawaki T, et al. Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr J 2009;56(9):1103–1111.
Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, et al. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol 2007;193(1):65–74.
Cunha DA, Ladriere L, Ortis F, Esteve MI, Gurzov EN, Lupi R, et al. Glucagon-Like Peptide-1 Agonists Protect Pancreatic β-Cells From Lipotoxic Endoplasmic Reticulum Stress Through Upregulation of BiP and JunB. Diabetes 2009;58:2851–2862.
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313(5790):1137–1140.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balasubramanyam, M., Lenin, R. & Monickaraj, F. Endoplasmic reticulum stress in diabetes: New insights of clinical relevance. Indian J Clin Biochem 25, 111–118 (2010). https://doi.org/10.1007/s12291-010-0022-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-010-0022-1