Abstract
Background
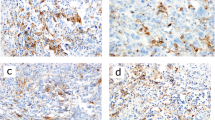
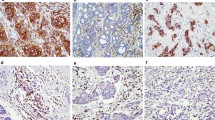
B7 homolog 4 (B7-H4) and indoleamine 2,3-dioxygenase (IDO1) are factors involved in the inhibition of antitumor activity and are new therapeutic targets for immune checkpoint therapy. Our study aimed to simultaneously investigate the interrelationship among B7-H4, IDO1 and programmed cell death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC), including tumor immune microenvironment (TIME) and TNBC subtypes.
Methods
Immunostaining for PD-L1, B7-H4, and IDO1 was performed on whole-slide sections of 119 cases of TNBC. The TIME was evaluated based on stromal tumor infiltrating lymphocytes (sTILs; %), pattern classification of TILs, tumor–stroma ratio (TSR), and tertiary lymphoid structure (TLS). TNBC subtypes were also determined by immunohistochemistry analysis of cytokeratin 5/6 and androgen receptor (AR) expression.
Results
B7-H4 expression was significantly higher in cases with a combined positive score cutoff of 5 for PD-L1 (clone 28–8; p = 0.021), inflamed TIL pattern (p = 0.007), and TLS ≥ 4 (p = 0.006). B7-H4 expression was higher in case of CK5/6 ≥ 10 (p = 0.035). The H-scores of AR and B7-H4 were inversely correlated (ρ = − 0.509, p < 0.001). B7-H4 and IDO1 expression levels were inversely correlated in cases with AR < 10 (ρ = − 0.354, p < 0.001).
Conclusions
These results suggest that considering the TIL pattern and TLS and identifying the expression of PD-L1 and the basal-like type are useful for estimating B7-H4 expression. In addition, luminal androgen receptor (LAR)-type is frequently deficient in B7-H4 expression. In non-LAR types, B7-H4 and IDO1 expression are exclusive.





Similar content being viewed by others

Data availability
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
References
Iacopetta D, Ceramella J, Baldino N, Sinicropi MS, Catalano A. Targeting breast cancer: an overlook on current strategies. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24043643.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28. https://doi.org/10.1016/S0140-6736(20)32531-9.
Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, et al. The immunomodulatory protein B7–H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–41. https://doi.org/10.1016/j.yexcr.2005.01.018.
Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–61. https://doi.org/10.1016/s1074-7613(03)00152-3.
Podojil JR, Miller SD. Potential targeting of B7–H4 for the treatment of cancer. Immunol Rev. 2017;276:40–51. https://doi.org/10.1111/imr.12530.
Kinneer K, Wortmann P, Cooper ZA, Dickinson NJ, Masterson L, Cailleau T, et al. Design and preclinical evaluation of a novel B7–H4-directed antibody-drug conjugate, AZD8205, alone and in combination with the PARP1-selective inhibitor AZD5305. Clin Cancer Res. 2023;29:1086–101. https://doi.org/10.1158/1078-0432.CCR-22-2630.
Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ. Indoleamine 2,3- dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol. 2018;336:175–203. https://doi.org/10.1016/bs.ircmb.2017.07.004.
Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, et al. Indoleamine 2,3- dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022. https://doi.org/10.1016/j.ctrv.2022.102461.
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–97. https://doi.org/10.1016/S1470-2045(19)30274-8.
Kjeldsen JW, Lorentzen CL, Martinenaite E, Ellebaek E, Donia M, Holmstroem RB, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med. 2021;27:2212–23. https://doi.org/10.1038/s41591-021-01544-x.
Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, et al. Differential expression and significance of PD-L1, IDO-1, and B7–H4 in human lung cancer. Clin Cancer Res. 2017;23:370–8. https://doi.org/10.1158/1078-0432.CCR-16-0150.
O’Meara TA, Tolaney SM. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget. 2021;12:394–400. https://doi.org/10.18632/oncotarget.27877.
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661–72. https://doi.org/10.1016/j.annonc.2021.02.006.
El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, et al. The tale of TILs in breast cancer: a report from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer. 2021;7:150. https://doi.org/10.1038/s41523-021-00346-1.
Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365–86. https://doi.org/10.7150/thno.58390.
Vaghjiani RG, Skitzki JJ. Tertiary lymphoid structures as mediators of immunotherapy response. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14153748.
Hagenaars SC, Vangangelt KMH, Van Pelt GW, Karancsi Z, Tollenaar RAEM, Green AR, et al. Standardization of the tumor-stroma ratio scoring method for breast cancer research. Breast Cancer Res Treat. 2022;193:545–53. https://doi.org/10.1007/s10549-022-06587-3.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. https://doi.org/10.1172/JCI45014.
Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS One. 2016. https://doi.org/10.1371/journal.pone.0157368.
Kumar S, Bal A, Das A, Bhattacharyya S, Laroiya I, Khare S, et al. Molecular subtyping of triple negative breast cancer by surrogate immunohistochemistry markers. Appl Immunohistochem Mol Morphol. 2021;29:251–7. https://doi.org/10.1097/PAI.0000000000000897.
Kim S, Moon BI, Lim W, Park S, Cho MS, Sung SH. Feasibility of classification of triple negative breast cancer by immunohistochemical surrogate markers. Clin Breast Cancer. 2018. https://doi.org/10.1016/j.clbc.2018.03.012.
Choupani E, Mahmoudi Gomari M, Zanganeh S, Nasseri S, Haji-Allahverdipoor K, Rostami N, et al. Newly developed targeted therapies against the androgen receptor in triple-negative breast cancer: a review. Pharmacol Rev. 2023;75:309–27. https://doi.org/10.1124/pharmrev.122.000665.
Huang X, Ding Q, Guo H, Gong Y, Zhao J, Zhao M, et al. Comparison of three FDA- approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum Pathol. 2021;108:42–50. https://doi.org/10.1016/j.humpath.2020.11.004.
Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27:92–100. https://doi.org/10.1097/PAI.0000000000000594.
Vtorushin S, Dulesova A, Krakhmal N. Luminal androgen receptor (LAR) subtype of triple-negative breast cancer: Molecular, morphological, and clinical features. J Zhejiang Univ Sci B. 2022;23:617–24. https://doi.org/10.1631/jzus.B2200113.
Hashmi AA, Naz S, Hashmi SK, Hussain ZF, Irfan M, Bakar SMA, et al. Cytokeratin 5/6 and cytokeratin 8/18 expression in triple negative breast cancers: Clinicopathologic significance in South-Asian population. BMC Res Notes. 2018;11:372. https://doi.org/10.1186/s13104-018-3477-4.
Sofopoulos M, Fortis SP, Vaxevanis CK, Sotiriadou NN, Arnogiannaki N, Ardavanis A, et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol Immunother. 2019;68:1733–45. https://doi.org/10.1007/s00262-019-02407-8.
Altan M, Kidwell KM, Pelekanou V, Carvajal-Hausdorf DE, Schalper KA, Toki MI, et al. Association of B7–H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer. NPJ Breast Cancer. 2018;4:40. https://doi.org/10.1038/s41523-018-0095-1.
Alkhayyal N, Elemam NM, Hussein A, Magdub S, Jundi M, Maghazachi AA, et al. (2022) Expression of immune checkpoints (PD-L1 and IDO) and tumour-infiltrating lymphocytes in breast cancer. Heliyon 10.1016 j.heliyon.2022.e10482
Wang L, Yang C, Liu XB, Wang L, Kang FB. B7–H4 overexpression contributes to poor prognosis and drug-resistance in triple-negative breast cancer. Cancer Cell Int. 2018;18:100. https://doi.org/10.1186/s12935-018-0597-9.
Kim S, Park S, Cho MS, Lim W, Moon BI, Sung SH. Strong correlation of indoleamine 2,3-dioxygenase 1 expression with basal-like phenotype and increased lymphocytic infiltration in triple-negative breast cancer. J Cancer. 2017;8:124–30. https://doi.org/10.7150/jca.17437.
Kim NI, Park MH, Cho N, Lee JS. Comparison of the clinicopathologic features and T-cell infiltration of B7–H3 and B7–H4 expression in triple-negative breast cancer subtypes. Appl Immunohistochem Mol Morphol. 2022;30:246–56. https://doi.org/10.1097/PAI.0000000000001001.
Khan M, Du K, Ai M, Wang B, Lin J, Ren A, et al. PD-L1 expression as biomarker of efficacy of PD-1/PD-L1 checkpoint inhibitors in metastatic triple negative breast cancer: a systematic review and meta-analysis. Front Immunol. 2023;14:1060308. https://doi.org/10.3389/fimmu.2023.1060308.
Acknowledgements
We thank Mrs. Kaoru Tsuboi, Mrs. Megumi Kuriyama, and Mr. Nobuhisa Iwachidou for their technical assistance.
Funding
This work was supported by JSPS KAKENHI (Grant number 21K06934) and Research Project Grants from Kawasaki Medical School (R04G002).
Author information
Authors and Affiliations
Contributions
This study was designed and conceived by FS and TM. The material was prepared by Yuka- M, YF, and FS. Data collection and statistical analysis were performed by FS. The stained specimens were evaluated by FS and TM. Technical input was provided by NH, Yuka-M, and Yasumasa-M; Clinical information was obtained by NN and NT. The first draft of the manuscript was written by FS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was conducted after obtaining approval from the Ethics Committee of Kawasaki Medical School and in accordance with the Declaration of Helsinki (approval number 5013–03).
Informed consent
Although this was a retrospective study, each patient provided informed consent for study inclusion before surgery. A summary of the study protocol was published on a web page, which explained the study and provided an opportunity for dissenting opinions to be expressed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sanuki, F., Mikami, Y., Nishimura, H. et al. Immunohistological analysis of B7-H4, IDO1, and PD-L1 expression and tumor immune microenvironment based on triple-negative breast cancer subtypes. Breast Cancer 30, 1041–1053 (2023). https://doi.org/10.1007/s12282-023-01498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01498-7