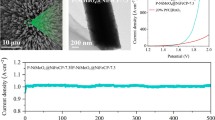
Abstract
The urea oxidation reaction has attracted increasing attention. Here, porous rod-like Ni2P/Ni assemblies, which consist of numerous nanoparticle subunits with matching interfaces at the nanoscale have been synthesized via a simple phosphating approach. Density functional theory calculations and density of states indicate that porous rod-like Ni2P/Ni assemblies can significantly enhance the activity of chemical bonds and the conductivity compared with NiO/Ni toward the urea oxidation reaction. The optimal catalyst of Ni2P/Ni can deliver a low overpotential of 50 mV at 10 mA·cm−2 and Tafel slope of 87.6 mV·dec−1 in urea oxidation reaction. Moreover, the constructed electrolytic cell exhibits a current density of 10 mA·cm−2 at a cell voltage of 1.47 V and an outstanding durability in the two-electrode system. This work has provided a new possibility to fabricate metal phosphides-metal assemblies with advanced performance.

Similar content being viewed by others
References
Ma, B.; Yang, Z. C.; Chen, Y. T.; Yuan, Z. H. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380.
Wang, T. T.; Wang, M.; Yang, H.; Xu, M. Q.; Zuo, C. D.; Feng, K.; Xie, M.; Deng, J.; Zhong, J.; Zhou, W. et al. Weakening hydrogen adsorption on nickel via interstitial nitrogen doping promotes bifunctional hydrogen electrocatalysis in alkaline solution. Energy Environ. Sci. 2019, 12, 3522–3529.
Xie, C.; Yan, D. F.; Chen, W.; Zou, Y. Q.; Chen, R.; Zang, S. Q.; Wang, Y. Y.; Yao, X. D.; Wang, S. Y. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68.
Sun, K. A.; Zhao, L.; Zeng, L. Y.; Liu, S. J.; Zhu, H. Y.; Li, Y. P.; Chen, Z.; Zhuang, Z. W.; Li, Z. L.; Liu, Z. et al. Reaction environment self-modification on low-coordination Ni2+ octahedra atomic interface for superior electrocatalytic overall water splitting. Nano Res. 2020, 13, 3068–3074.
Ge, J.; Zhang, W.; Tu, J.; Xia, T.; Chen, S. P.; Xie, G. Suppressed Jahn-Teller distortion in MnCo2O4@Ni2P heterostructures to promote the overall water splitting. Small, 2020, 16, 2001856.
Li, C. C.; Liu, Y. W.; Zhuo, Z. W.; Ju, H. X.; Li, D. A.; Guo, Y. P.; Wu, X. J.; Li, H. Q.; Zhai, T. Y. Local charge distribution engineered by Schottky heterojunctions toward urea electrolysis. Adv. Energy Mater. 2018, 8, 1801775.
Wang, X. X.; Wang, J. M.; Sun, X. P.; Wei, S.; Cui, L.; Yang, W. R.; Liu, J. Q. Hierarchical coral-like NiMoS nanohybrids as highly efficient bifunctional electrocatalysts for overall urea electrolysis. Nano Res. 2018, 11, 988–996.
Zhang, H.; Li, H. Y.; Akram, B.; Wang, X. Fabrication of NiFe layered double hydroxide with well-defined laminar superstructure as highly efficient oxygen evolution electrocatalysts. Nano Res. 2019, 12, 1327–1331.
Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921.
Wang, L.; Zhu, S. Q.; Marinkovic, N.; Kattel, S.; Shao, M. H.; Yang, B. L.; Chen, J. G. Insight into the synergistic effect between nickel and tungsten carbide for catalyzing urea electrooxidation in alkaline electrolyte. Appl. Catal. B Environ. 2018, 232, 365–370.
Xiao, X.; Zou, L. L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal-organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331.
Tang, W. K.; Liu, X. F.; Li, Y.; Pu, Y. H.; Lu, Y.; Song, Z. M.; Wang, Q.; Yu, R. H.; Shui, J. Boosting electrocatalytic water splitting via metal-metalloid combined modulation in quaternary Ni-Fe-P-B amorphous compound. Nano Res. 2020, 13, 447–454.
Wang, J. M.; Kong, R. M.; Asiri, A. M.; Sun, X. P. Replacing oxygen evolution with hydrazine oxidation at the anode for energy-saving electrolytic hydrogen production. ChemElectroChem 2017, 4, 481–484.
Wei, C.; Sun, S. N.; Mandler, D.; Wang, X.; Qiao, S. Z.; Xu, Z. J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534.
Yang, D. W.; Gu, Y.; Yu, X.; Lin, Z. X.; Xue, H. G.; Feng, L. G. Nanostructured Ni2P-C as an efficient catalyst for urea electrooxidation. ChemElectroChem 2018, 5, 659–664.
Yan, L.; Sun, Y. L.; Hu, E. L.; Ning, J. Q.; Zhong, Y. J.; Zhang, Z. Y.; Hu, Y. Facile in-situ growth of Ni2P/Fe2P nanohybrids on Ni foam for highly efficient urea electrolysis. J. Colloid Interface Sci. 2019, 541, 279–286.
Zhu, B. J.; Liang, Z. B.; Zou, R. Q. Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small 2020, 16, 1906133.
Liu, D. N.; Liu, T. T.; Zhang, L. X.; Qu, F. L.; Du, G.; Asiri, A. M.; Sun, X. P. High-performance urea electrolysis towards less energy-intensive electrochemical hydrogen production using a bifunctional catalyst electrode. J. Mater. Chem. A 2017, 5, 3208–3213.
Tang, C.; Zhang, R.; Lu, W. B.; Wang, Z.; Liu, D. N.; Hao, S.; Du, G.; Asiri, A. M.; Sun, X. P. Energy-saving electrolytic hydrogen generation: Ni2P nanoarray as a high-performance non-noble-metal electrocatalyst. Angew. Chem., Int. Ed. 2017, 56, 842–846.
Liu, X.; Ni, K.; Wen, B.; Guo, R. T.; Niu, C. J.; Meng, J. S.; Li, Q.; Wu, P. J.; Zhu, Y. W.; Wu, X. J. et al. Deep reconstruction of nickel-based precatalysts for water oxidation catalysis. ACS Energy Lett. 2019, 4, 2585–2592.
Tong, Y.; Chen, P. Z.; Zhang, M. X.; Zhou, T. P.; Zhang, L. D.; Chu, W. S.; Wu, C. Z.; Xie, Y. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal. 2018, 8, 1–7.
Yu, Z. Y.; Duan, Y.; Gao, M. R.; Lang, C. C.; Zheng, Y. R.; Yu, S. H. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973.
Meguerdichian, A. G.; Jafari, T.; Shakil, M. R.; Miao, R.; Achola, L. A.; MacHaria, J.; Shirazi-Amin, A.; Suib, S. L. Synthesis and electrocatalytic activity of ammonium nickel phosphate, [NH4]NiPO4·6H2O, and β-nickel pyrophosphate, β-Ni2P2O7: Catalysts for electrocatalytic decomposition of urea. Inorg. Chem. 2018, 57, 1815–1823.
Peng, C. Y.; Kang, L.; Cao, S.; Chen, Y.; Lin, Z. S.; Fu, W. F. Nanostructured Ni2P as a robust catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angew. Chem., Int. Ed. 2015, 54, 15725–15729.
Luo, Z. Z.; Zhang, Y.; Zhang, C. H.; Tan, H. T.; Li, Z.; Abutaha, A.; Wu, X. L.; Xiong, Q. H.; Khor, K. A.; Hippalgaonkar, K. et al. Multifunctional 0D-2D Ni2P nanocrystals-black phosphorus heterostructure. Adv. Energy Mater. 2017, 7, 1601285.
Wen, J.; Feng, Z.; Liu, H. R.; Chen, T.; Yang, Y. Q.; Li, S. Z.; Sheng, S.; Fang, G. J. In-situ synthesized Ni2P nanosheet arrays as the cathode for novel alkaline Ni//Zn rechargeable battery. Appl. Surf. Sci. 2019, 485, 462–467.
An, C. H.; Wang, Y. J.; Wang, Y. P.; Liu, G.; Li, L.; Qiu, F. Y.; Xu, Y. N.; Jiao, L. F.; Yuan, H. T. Facile synthesis and superior supercapacitor performances of Ni2P/RGO nanoparticles. RSC Adv. 2013, 3, 4628–4633.
Jiang, P.; Liu, Q.; Sun, X. P. NiP2 nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions. Nanoscale 2014, 6, 13440–13445.
Hao, S.; Yang, L. B.; Liu, D. N.; Kong, R. M.; Du, G.; Asiri, A. M.; Yang, Y. C.; Sun, X. P. Integrating natural biomass electro-oxidation and hydrogen evolution: Using a porous Fe-doped CoP nanosheet array as a bifunctional catalyst. Chem. Commun. 2017, 53, 5710–5713.
Wang, J. M.; Ma, X.; Liu, T. T.; Liu, D. N.; Hao, S.; Du, G.; Kong, R. M.; Asiri, A. M.; Sun, X. P. NiS2 nanosheet array: A high-active bifunctional electrocatalyst for hydrazine oxidation and water reduction toward energy-efficient hydrogen production. Mater. Today Energy 2017, 3, 9–14.
Yang, F.; Ye, J. Y.; Yuan, Q.; Yang, X. T.; Xie, Z. X.; Zhao, F. L.; Zhou, Z. Y.; Gu, L.; Wang, X. Ultrasmall Pd-Cu-Pt trimetallic twin icosahedrons boost the electrocatalytic performance of glycerol oxidation at the operating temperature of fuel cells. Adv. Funct. Mater. 2020, 30, 1908235.
Ni, B.; Zhang, Q. H.; Ouyang, C.; Zhang, S. M.; Yu, B.; Zhuang, J.; Gu, L.; Wang, X. The synthesis of sub-nano-thick Pd nanobelt-based materials for enhanced hydrogen evolution reaction activity. CCS Chem. 2020, 2, 642–654.
Zhu, D. D.; Guo, C. X.; Liu, J. L.; Wang, L.; Du, Y.; Qiao, S. Z. Two-dimensional metal-organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 2017, 53, 10906–10909.
Xu, Y.; Chai, X. J.; Ren, T. L.; Yu, S. S.; Yu, H. J.; Wang, Z. Q.; Li, X. N.; Wang, L.; Wang, H. J. Ir-doped Ni-based metal-organic framework ultrathin nanosheets on Ni foam for enhanced urea electro-oxidation. Chem. Commun. 2020, 56, 2151–2154.
Lan, R.; Tao, S. W.; Irvine, J. T. S. A direct urea fuel cell—Power from fertiliser and waste. Energy Environ. Sci. 2010, 3, 438–441.
Ding, R.; Qi, L.; Jia, M. J.; Wang, H. Y. Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2014, 6, 1369–1376.
Chen, S.; Duan, J. J.; Vasileff, A.; Qiao, S. Z. Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angew. Chem., Int. Ed. 2016, 55, 3804–3808.
Liang, Y. H.; Liu, Q.; Asiri, A. M.; Sun, X. P. Enhanced electrooxidation of urea using NiMoO4·xH2O nanosheet arrays on Ni foam as anode. Electrochim. Acta 2015, 153, 456–460.
Guo, F.; Ye, K.; Du, M. M.; Huang, X. M.; Cheng, K.; Wang, G. L.; Cao, D. X. Electrochemical impedance analysis of urea electrooxidation mechanism on nickel catalyst in alkaline medium. Electrochim. Acta 2016, 210, 474–482.
Sha, L. N.; Ye, K.; Wang, G.; Shao, J. Q.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G. L.; Cao, D. X. Hierarchical NiCo2O4 nanowire array supported on Ni foam for efficient urea electrooxidation in alkaline medium. J. Power Sources 2019, 412, 265–271.
Zeng, M.; Wu, J. H.; Li, Z. Y.; Wu, H. H.; Wang, J. L.; Wang, H. L.; He, L.; Yang, X. J. Interlayer effect in NiCo layered double hydroxide for promoted electrocatalytic urea oxidation. ACS Sustainable Chem. Eng. 2019, 7, 4777–4783.
Sun, Y. F.; Gao, S.; Lei, F. C.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636.
Li, D. D.; Xu, H. Q.; Jiao, L.; Jiang, H. L. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. EnergyChem 2019, 7, 100005.
Liu, Q. D.; Wang, X. Polyoxometalate clusters: Sub-nanometer building blocks for construction of advanced materials. Matter 2020, 2, 816–841.
Liu, M.; Zhang, R.; Zhang, L. X.; Liu, D. N.; Hao, S.; Du, G.; Asiri, A. M.; Kong, R. M.; Sun, X. P. Energy-efficient electrolytic hydrogen generation using a Cu3P nanoarray as a bifunctional catalyst for hydrazine oxidation and water reduction. Inorg. Chem. Front. 2017, 4, 420–423.
Xie, Y. N.; Zhang, Z. Y.; Zhong, D. L.; Peng, L. M. Speeding up carbon nanotube integrated circuits through three-dimensional architecture. Nano Res. 2019, 12, 1810–1816.
Zhang, S. M.; Shi, W. X.; Rong, S. J.; Li, S. Z.; Zhuang, J.; Wang, X. Chirality evolution from sub-1 nanometer nanowires to the macroscopic helical structure. J. Am. Chem. Soc. 2020, 142, 1375–1381.
Li, Q.; Li, N.; An, J.; Pang, H. Controllable synthesis of a mesoporous NiO/Ni nanorod as an excellent catalyst for urea electro-oxidation. Inorg. Chem. Front. 2020, 7, 2089–2096.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Wexler, R. B.; Martirez, J. M. P.; Rappe, A. M. Active role of phosphorus in the hydrogen evolving activity of nickel phosphide (0001) surfaces. ACS Catal. 2017, 7, 7718–7725.
Yuan, Y.; Dong, X. Q.; Ricardez-Sandoval, L. A density functional theory analysis on syngas adsorption on NiO (100) surface. Appl. Surf. Sci. 2019, 498, 143782.
Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y.; Humphreys, C. J.; Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509.
Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J. R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
Peterson, A. A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315.
Zhou, K.; Zhou, W. J.; Yang, L. J.; Lu, J.; Cheng, S.; Mai, W. J.; Tang, Z. H.; Li, L. G.; Chen, S. W. Ultrahigh-performance pseudocapacitor electrodes based on transition metal phosphide nanosheets array via phosphorization: A general and effective approach. Adv. Funct. Mater. 2015, 25, 7530–7538.
Wang, G.; Ye, K.; Shao, J. Q.; Zhang, Y. Y.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G. L.; Cao, D. X. Porous Ni2P nanoflower supported on nickel foam as an efficient three-dimensional electrode for urea electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2018, 43, 9316–9325.
Lin, Y.; He, L. B.; Chen, T.; Zhou, D.; Wu, L.; Hou, X. D.; Zheng, C. B. Cost-effective and environmentally friendly synthesis of 3D Ni2P from scrap nickel for highly efficient hydrogen evolution in both acidic and alkaline media. J. Mater. Chem. A 2018, 6, 4088–4094.
Miao, Y. Q.; Ouyang, L.; Zhou, S. L.; Xu, L. N.; Yang, Z. Y.; Xiao, M. S.; Ouyang, R. Z. Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosens. Bioelectron. 2014, 53, 428–439.
Vedharathinam, V.; Botte, G. G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665.
Zhang, X.; Liu, Y. Y.; Xiong, Q. Z.; Liu, G. Q.; Zhao, C. J.; Wang, G. Z.; Zhang, Y. X.; Zhang, H. M.; Zhao, H. J. Vapour-phase hydrothermal synthesis of Ni2P nanocrystallines on carbon fiber cloth for high-efficiency H2 production and simultaneous urea decomposition. Electrochim. Acta 2017, 254, 44–49.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. U1904215 and 21671170), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and Qinglan Project of Jiangsu and Program for Colleges Natural Science Research in Jiangsu Province (No. 18KJB150036). We also acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, Q., Li, X., Gu, J. et al. Porous rod-like Ni2P/Ni assemblies for enhanced urea electrooxidation. Nano Res. 14, 1405–1412 (2021). https://doi.org/10.1007/s12274-020-3190-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3190-1