Abstract
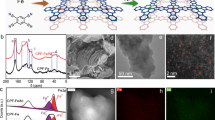
An anti-oxidized NiS2 electrocatalyst with improved catalytic activity was developed using a Fe-induced conversion strategy. X-ray photoelectron spectroscopy reveals that betatopic Ni species with high valence states are present within the Fe-NiS2 matrix and relatively less oxidized layers exist on the catalyst’s surface, indicating its greatly enhanced anti-oxidized capability. Density functional theory calculations reveal that the Ni and Fe sites on the Fe-NiS2 catalyst surface possess strong adsorption capacity toward hydroxyl ions compared with the Ni sites on NiS2. Benefiting from its unique microstructure and modulated electronic structure due to the effects of iron species, the Fe-NiS2 catalyst prepared on carbon fiber delivers a remarkably enhanced catalytic activity and superior long-life durability for overall water splitting. The present results provide an efficient strategy for the design and configuration of anti-oxidation catalysts, especially for energy storage and catalysis.

Similar content being viewed by others
References
Lewis, N. S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920.
Hu, C. G.; Chen, X. Y.; Dai, Q. B.; Wang, M.; Qu, L. T.; Dai, L. M. Earth-abundant carbon catalysts for renewable generation of clean energy from sunlight and water. Nano Energy 2017, 41, 367–376.
Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086.
Zou, X. X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180.
Hu, C. G.; Dai, L. M. Multifunctional carbon-based metal-free electrocatalysts for simultaneous oxygen reduction, oxygen evolution, and hydrogen evolution. Adv. Mater. 2017, 29, 1604942.
Peng, Z.; Jia, D. S.; Al-Enizi, A. M.; Elzatahry, A. A.; Zheng, G. F. From water oxidation to reduction: Homologous Ni–Co based nanowires as complementary water splitting electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031.
Zhu, X. L.; Tang, C.; Wang, H. F.; Li, B. Q.; Zhang, Q.; Li, C. Y.; Yang, C. H.; Wei, F. Monolithic-structured ternary hydroxides as freestanding bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 7245–7250.
Ouyang, C. B.; Wang, X.; Wang, C.; Zhang, X. X.; Wu, J. H.; Ma, Z. L.; Dou, S.; Wang, S. Y. Hierarchically porous Ni3S2 nanorod array foam as highly efficient electrocatalyst for hydrogen evolution reaction and oxygen evolution reaction. Electrochim. Acta 2015, 174, 297–301.
Feng, L. L.; Yu, G. T.; Wu, Y. Y.; Li, G. D.; Li, H.; Sun, Y. H.; Asefa, T.; Chen, W.; Zou, X. X. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026.
Yu, L.; Xia, B. Y.; Wang, X.; Lou, X. W. General formation of M-MoS3 (M = Co, Ni) hollow structures with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 2016, 28, 92–97.
Stern, L. A.; Feng, L. G.; Song, F.; Hu, X. L. Ni2P as a janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351.
Feng, Y. Y.; Ouyang, Y.; Peng, L.; Qiu, H. J.; Wang, H. L.; Wang, Y. Quasi-graphene-envelope Fe-doped Ni2P sandwiched nanocomposites for enhanced water splitting and lithium storage performance. J. Mater. Chem. A 2015, 3, 9587–9594.
Huang, H. W.; Yu, C.; Yang, J.; Han, X. T.; Zhao, C. T.; Li, S. F.; Liu, Z. B.; Qiu, J. S. Ultrasmall diiron phosphide nanodots anchored on graphene sheets with enhanced electrocatalytic activity for hydrogen production via high-efficiency water splitting. J. Mater. Chem. A 2016, 4, 16028–16035.
Xie, J. F.; Xie, Y. Transition metal nitrides for electrocatalytic energy conversion: Opportunities and challenges. Chem.—Eur. J. 2016, 22, 3588–3598.
Song, C. S. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263.
Tian, S.; Li, X.; Wang, A. J.; Prins, R.; Chen, Y. Y.; Hu, Y. K. Facile preparation of Ni2P with a sulfur-containing surface layer by low-temperature reduction of Ni2P2S6. Angew. Chem., Int. Ed. 2016, 55, 4030–4034.
Oyama, S. T.; Gott, T.; Zhao, H. Y.; Lee, Y. K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107.
Lu, Z. Y.; Xu, W. W.; Zhu, W.; Yang, Q.; Lei, X. D.; Liu, J. F.; Li, Y. P.; Sun, X. M.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482.
Yu, C.; Liu, Z. B.; Han, X. T.; Huang, H. W.; Zhao, C. T.; Yang, J.; Qiu, J. S. NiCo-layered double hydroxides vertically assembled on carbon fiber papers as binder-free high-active electrocatalysts for water oxidation. Carbon 2016, 110, 1–7.
Huang, H. W.; Yu, C.; Zhao, C. T.; Han, X. T.; Yang, J.; Liu, Z. B.; Li, S. F.; Zhang, M. D.; Qiu, J. S. Iron-tuned super nickel phosphide microstructures with high activity for electrochemical overall water splitting. Nano Energy 2017, 34, 472–480.
Tang, C.; Pu, Z. H.; Liu, Q.; Asiri, A. M.; Sun, X. P. NiS2 nanosheets array grown on carbon cloth as an efficient 3D hydrogen evolution cathode. Electrochim. Acta 2015, 153, 508–514.
Wan, C.; Leonard, B. M. Iron-doped molybdenum carbide catalyst with high activity and stability for the hydrogen evolution reaction. Chem. Mater. 2015, 27, 4281–4288.
Cheng, N. Y.; Liu, Q.; Asiri, A. M.; Xing, W.; Sun, X. P. A Fe-doped Ni3S2 particle film as a high-efficiency robust oxygen evolution electrode with very high current density. J. Mater. Chem. A 2015, 3, 23207–23212.
Huang, Z. F.; Song, J. J.; Li, K.; Tahir, M.; Wang, Y. T.; Pan, L.; Wang, L.; Zhang, X. W.; Zou, J. J. Hollow cobaltbased bimetallic sulfide polyhedra for efficient all-pH-value electrochemical and photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2016, 138, 1359–1365.
Faber, M. S.; Lukowski, M. A.; Ding, Q.; Kaiser, N. S.; Jin, S. Earth-abundant metal pyrites (FeS2, CoS2, NiS2, and their alloys) for highly efficient hydrogen evolution and polysulfide reduction electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356.
Yang, S. L.; Yao, H. B.; Gao, M. R.; Yu, S. H. Monodisperse cubic pyrite NiS2 dodecahedrons and microspheres synthesized by a solvothermal process in a mixed solvent: Thermal stability and magnetic properties. CrystEngComm 2009, 11, 1383–1390.
Kibsgaard, J.; Tsai, C.; Chan, K. R.; Benck, J. D.; Nørskov, J. K.; Abild-Pedersen, F.; Jaramillo, T. F. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029.
Wang, D. Y.; Gong, M.; Chou, H. L.; Pan, C. J.; Chen, H. A.; Wu, Y. P.; Lin, M. C.; Guan, M. Y.; Yang, J.; Chen, C. W. et al. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets-carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592.
Yang, N.; Tang, C.; Wang, K. Y.; Du, G.; Asiri, A. M.; Sun, X. P. Iron-doped nickel disulfide nanoarray: A highly efficient and stable electrocatalyst for water splitting. Nano Res. 2016, 9, 3346–3354.
Lu, X. Y.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616.
Jia, X. D.; Zhao, Y. F.; Chen, G. B.; Shang, L.; Shi, R.; Kang, X. F.; Waterhouse, G. I. N.; Wu, L. Z.; Tung, C. H.; Zhang, T. R. Ni3FeN nanoparticles derived from ultrathin nife-layered double hydroxide nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585.
Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R. H.; Liu, S. H.; Zhuang, X. D.; Feng, X. L. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem., Int. Ed. 2016, 55, 6702–6707.
Li, J.; Wang, Y. C.; Zhou, T.; Zhang, H.; Sun, X. H.; Tang, J.; Zhang, L. J.; Al-Enizi, A. M.; Yang, Z. Q.; Zheng, G. F. Nanoparticle superlattices as efficient bifunctional electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14305–14312.
Nørskov, J. K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943.
Lu, Z. Y.; Zhu, W.; Yu, X. Y.; Zhang, H. C.; Li, Y. J.; Sun, X. M.; Wang, X. W.; Wang, H.; Wang, J. M.; Luo, J. et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (Nos. 21522601, U1508201, and 21361162004), the National Key Research and Development Program of China (No. 2016YFB0101201), and the Fundamental Research Funds for the Central Universities (No. DUT17LAB18).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yu, C., Huang, H., Zhou, S. et al. An electrocatalyst with anti-oxidized capability for overall water splitting. Nano Res. 11, 3411–3418 (2018). https://doi.org/10.1007/s12274-017-1964-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1964-x