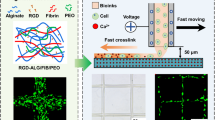
Abstract
Alginate is a widely used hydrogel in tissue engineering owing to its simple and non-cytotoxic gelation process, ease of use, and abundance. However, unlike hydrogels derived from mammalian sources such as collagen, alginate does not contain cell adhesion ligands. Here, we present a novel laser ablation technique for the in situ embedding of gold and iron nanoparticles into hydrogels. We hypothesized that integration of metal nanoparticles in alginate could serve as an alternative material because of its chemical biofunctionalization ability (coupling of RGD ligands) to favor cell adhesion. Cytocompatibility and biofunctionality of the gels were assessed by cell culture experiments using fibroblasts and endothelial cells. Nanoparticles with an average particle size of 3 nm (gold) and 6 nm (iron) were generated and stably maintained in alginate for up to 6 months. Using an extrusion system, several centimeter-long alginate tubes with an outer diameter of approximately 3 mm and a wall thickness of approximately 150 μm were manufactured. Confocal microscopy revealed homogeneously distributed nanoparticle agglomerates over the entire tube volume. Endothelial cells seeded on iron-loaded gels showed significantly higher viability and an increased degree of spreading, and the number of attached cells was also elevated in comparison to the control and gold-loaded alginates. We conclude that laser-based in situ integration of iron nanoparticles (⩽0.01 wt.%) in alginate is a straightforward method to generate composite materials that favor the adhesion of endothelial cells. In addition, we show that nanoparticle integration does not impair the alginate’s gelation and 3D biofabrication properties.

Similar content being viewed by others
References
Langer, R.; Vacanti, J. P. Tissue engineering. Science 1993, 260, 920–926.
Vacanti, C. A.; Vacanti, J. P. The science of tissue engineering. Orthop. Clin. North Am. 2000, 31, 351–355.
Malda, J.; Visser, J.; Melchels, F. P.; Jüngst, T.; Hennink, W. E.; Dhert, W. J. A.; Groll, J.; Hutmacher, D. W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028.
Murphy, S. V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
Henkel, J.; Hutmacher, D. W. Design and fabrication of scaffold-based tissue engineering. BioNanoMaterials 2013, 14, 171–193.
Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
Drury, J. L.; Mooney, D. J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351.
Tritz, J.; Rahouadj, R.; de Isla, N.; Charif, N.; Pinzano, A.; Mainard, D.; Bensoussan, D.; Netter, P.; Stoltz, J.-F.; Benkirane-Jessel, N. et al. Designing a three-dimensional alginate hydrogel by spraying method for cartilage tissue engineering. Soft Matter 2010, 6, 5165–5174.
Kuo, C. K.; Ma, P. X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521.
Drury, J. L.; Dennis, R. G.; Mooney, D. J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199.
Rowley, J. A.; Madlambayan, G.; Mooney, D. J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53.
Augst, A. D.; Kong, H. J.; Mooney, D. J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633.
Hess, C.; Schwenke, A.; Wagener, P.; Franzka, S.; Laszlo Sajti, C.; Pflaum, M.; Wiegmann, B.; Haverich, A.; Barcikowski, S. Dose-dependent surface endothelialization and biocompatibility of polyurethane noble metal nanocomposites. J. Biomed. Mater. Res. A 2014, 102, 1909–1920.
Hung, H. S.; Wu, C. C.; Chien, S.; Hsu, S. H. The behavior of endothelial cells on polyurethane nanocomposites and the associated signaling pathways. Biomaterials 2009, 30, 1502–1511.
Hsu, S. H.; Tang, C. M.; Tseng, H. J. Biocompatibility of poly(ether) urethane-gold nanocomposites. J. Biomed. Mater. Res. A 2006, 79, 759–770.
Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2010, 26, 2885–2893.
Anh, N. T.; Van Phu, D.; Duy, N. N.; Du, B. D.; Hien, N. Q. Synthesis of alginate stabilized gold nanoparticles by γ-irradiation with controllable size using different Au3+ concentration and seed particles enlargement. Radiat. Phys. Chem. 2010, 79, 405–408.
Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026.
Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821.
Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046.
Zhang, D. S.; Barcikowski, S. Rapid nanoparticle-polymer composites prototyping by laser ablation in liquids. In Encyclopedia of Polymeric Nanomaterials. Kobayashi, S.; Müllen, K., Eds.; Springer: Berlin Heidelberg, 2015; pp 2131–2141.
Rehbock, C.; Jakobi, J.; Gamrad, L.; van der Meer, S.; Tiedemann, D.; Taylor, U.; Kues, W.; Rath, D.; Barcikowski, S. Current state of laser synthesis of metal and alloy nanoparticles as ligand-free reference materials for nanotoxicological assays. Beilstein J. Nanotechnol. 2014, 5, 1523–1541.
Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381.
Chen, C.; Paw, B. H. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim. Biophys. Acta 2012, 1823, 1459–1467.
Koo, S. W.; Casper, K. A.; Otto, K. B.; Gira, A. K.; Swerlick, R. A. Iron chelators inhibit VCAM-1 expression in human dermal microvascular endothelial cells. J. Invest. Dermatol. 2003, 120, 871–879.
Wei, M. Q.; Wen, D. D.; Wang, X. Y.; Huan, Y.; Yang, Y.; Xu, J.; Cheng, K.; Zheng, M. W. Experimental study of endothelial progenitor cells labeled with superparamagnetic iron oxide in vitro. Mol. Med. Rep. 2015, 11, 3814–3819.
Horniblow, R. D.; Dowle, M.; Iqbal, T. H.; Latunde-Dada, G. O.; Palmer, R. E.; Pikramenou, Z.; Tselepis, C. Alginateiron speciation and its effect on in vitro cellular iron metabolism. PLoS One 2015, 10, e0138240.
Blaeser, A.; Campos, D. F. D.; Köpf, M.; Weber, M.; Fischer, H. Assembly of thin-walled, cell-laden hydrogel conduits inflated with perfluorocarbon. RSC Adv. 2014, 4, 46460–46469.
Wagener, P.; Schwenke, A.; Chichkov, B. N.; Barcikowski, S. Pulsed laser ablation of zinc in tetrahydrofuran: Bypassing the cavitation bubble. J. Phys. Chem. C 2010, 114, 7618–7625.
Tiedemann, D.; Taylor, U.; Rehbock, C.; Jakobi, J.; Klein, S.; Kues, W. A.; Barcikowski, S.; Rath, D. Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes. Analyst 2014, 139, 931–942.
Klein, S.; Petersen, S.; Taylor, U.; Rath, D.; Barcikowski, S. Quantitative visualization of colloidal and intracellular gold nanoparticles by confocal microscopy. J. Biomed. Opt. 2010, 15, 036015.
Jain, T. K.; Morales, M. A.; Sahoo, S. K.; Leslie-Pelecky, D. L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm. 2005, 2, 194–205.
Moreira, R.; Velz, T.; Alves, N.; Gesche, V. N.; Malischewski, A.; Schmitz-Rode, T.; Frese, J.; Jockenhoevel, S.; Mela, P. Tissue-engineered heart valve with a tubular leaflet design for minimally invasive transcatheter implantation. Tissue Eng. Part C Methods 2015, 21, 530–540.
Tsuji, T.; Thang, D. H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl. Surf. Sci. 2008, 254, 5224–5230.
Menéndez-Manjón, A.; Wagener, P.; Barcikowski, S. Transfer-matrix method for efficient ablation by pulsed laser ablation and nanoparticle generation in liquids. J. Phys. Chem. C 2011, 115, 5108–5114.
Jeon, J. S.; Yeh, C. S. Studies of silver nanoparticles by laser ablation method. J. Chin. Chem. Soc. 1998, 45, 721–726.
Blaeser, A.; Duarte Campos, D. F.; Puster, U.; Richtering, W.; Stevens, M. M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333.
Baladi, A.; Mamoory, R. S. Investigation of different liquid media and ablation times on pulsed laser ablation synthesis of aluminum nanoparticles. Appl. Surf. Sci. 2010, 256, 7559–7564.
Liu, Y. S.; Chen, S. M.; Zhong, L.; Wu, G. Z. Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255.
Petersen, S.; Jakobi, J.; Hörtinger, A.; Barcikowski, S. In-situ conjugation–tailored nanoparticle-conjugates by laser ablation in liquids. J. Laser Micro Nanoen. 2009, 4, 71–74.
Santra, S.; Kaittanis, C.; Grimm, J.; Perez, J. M. Drug/dyeloaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small 2009, 5, 1862–1868.
Hsu, S. H.; Tang, C. M.; Tseng, H. J. Gold nanoparticles induce surface morphological transformation in polyurethane and affect the cellular response. Biomacromolecules 2008, 9, 241–248.
Strauß, S.; Neumeister, A.; Barcikowski, S.; Kracht, D.; Kuhbier, J. W.; Radtke, C.; Reimers, K.; Vogt, P. M. Adhesion, vitality and osteogenic differentiation capacity of adipose derived stem cells seeded on nitinol nanoparticle coatings. PLoS One 2013, 8, e53309.
Wu, X.; Tan, Y.; Mao, H.; Zhang, M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomedicine 2010, 5, 385–399.
Apopa, P. L.; Qian, Y.; Shao, R.; Guo, N. L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X. L.; Vallyathan, V.; Castranova, V. et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part. Fibre Toxicol. 2009, 6, 1.
Gu, H. Y.; Chen, Z.; Sa, R. X.; Yuan, S. S.; Chen, H. Y.; Ding, Y. T.; Yu, A. M. The immobilization of hepatocytes on 24nm-sized gold colloid for enhanced hepatocytes proliferation. Biomaterials 2004, 25, 3445–3451.
Barcikowski, S.; Hahn, A.; Guggenheim, M.; Reimers, K.; Ostendorf, A. Biocompatibility of nanoactuators: Stem cell growth on laser-generated nickel–titanium shape memory alloy nanoparticles. J. Nanopart. Res. 2010, 12, 1733–1742.
Wagener, P.; Brandes, G.; Schwenke, A.; Barcikowski, S. Impact of in situ polymer coating on particle dispersion into solid laser-generated nanocomposites. Phys. Chem. Chem. Phys. 2011, 13, 5120–5126.
Li, K.; Schneider, M. Quantitative evaluation and visualization of size effect on cellular uptake of gold nanoparticles by multiphoton imaging-UV/Vis spectroscopic analysis. J. Biomed. Opt. 2014, 19, 101505.
Machida-Sano, I.; Matsuda, Y.; Namiki, H. In vitro adhesion of human dermal fibroblasts on iron cross-linked alginate films. Biomed. Mater. 2009, 4, 025008.
Ahmed, E. M.; Aggor, F. S. Swelling kinetic study and characterization of crosslinked hydrogels containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 117, 2168–2174.
Calderwood, D. A.; Shattil, S. J.; Ginsberg, M. H. Integrins and actin filaments: Reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 2000, 275, 22607–22610.
Pollard, T. D.; Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465.
Defilippi, P.; Olivo, C.; Venturino, M.; Dolce, L.; Silengo, L.; Tarone, G. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc. Res. Tech. 1999, 47, 67–78.
Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G. J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632.
Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J. F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011, 32, 9353–9363.
Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M. B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509.
Pozzi, D.; Caracciolo, G.; Digiacomo, L.; Colapicchioni, V.; Palchetti, S.; Capriotti, A. L.; Cavaliere, C.; Chiozzi, R. Z.; Puglisie, A.; Laganà, A. The biomolecular corona of nanoparticles in circulating biological media. Nanoscale 2015, 7, 13958–13966.
Michel, S. A. A. X.; Knetsch, M. L. W.; Koole, L. H. Adsorption of albumin on flax fibers increases endothelial cell adhesion and blood compatibility in vitro. J. Biomater. Sci. Polym. Ed. 2014, 25, 698–712.
Schwab, A. Function and spatial distribution of ion channels and transporters in cell migration. Am. J. Physiol. Renal. Physiol. 2001, 280, F739–F747.
Klausner, R. D.; Rouault, T. A.; Harford, J. B. Regulating the fate of mRNA: The control of cellular iron metabolism. Cell 1993, 72, 19–28.
Hentze, M. W.; Muckenthaler, M. U.; Andrews, N. C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297.
Li, J.; Lin, F. Microfluidic devices for studying chemotaxis and electrotaxis. Trends Cell Biol. 2011, 21, 489–497.
Machida-Sano, I.; Hirakawa, M.; Matsumoto, H.; Kamada, M.; Ogawa, S.; Satoh, N.; Namiki, H. Surface characteristics determining the cell compatibility of ionically cross-linked alginate gels. Biomed. Mater. 2014, 9, 025007.
Sowa-Söhle, E. N.; Schwenke, A.; Wagener, P.; Weiss, A.; Wiegel, H.; Sajti, C. L.; Haverich, A.; Barcikowski, S.; Loos, A. Antimicrobial efficacy, cytotoxicity, and ion release of mixed metal (Ag, Cu, Zn, Mg) nanoparticle polymer composite implant material. BioNanoMaterials 2013, 14, 217–227.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Electronic supplementary material
12274_2016_1218_MOESM1_ESM.pdf
Laser-based in situ embedding of metal nanoparticles into bioextruded alginate hydrogel tubes enhances human endothelial cell adhesion
Rights and permissions
About this article
Cite this article
Blaeser, A., Million, N., Campos, D.F.D. et al. Laser-based in situ embedding of metal nanoparticles into bioextruded alginate hydrogel tubes enhances human endothelial cell adhesion. Nano Res. 9, 3407–3427 (2016). https://doi.org/10.1007/s12274-016-1218-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1218-3