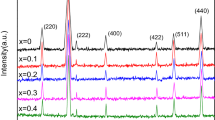
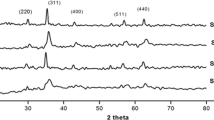
Abstract
Crystalline and nanostructured cobalt (CoFe2O4), nickel (NiFe2O4), zinc (ZnFe2O4) and manganese (MnFe2O4) spinel ferrites are synthesized with high yields, crystallinity and purity through an easy, quick, reproducible and low-temperature hydrothermal assisted route starting from an aqueous suspension of coprecipitated metal oxalates. The use of water as a reaction medium is a further advantage of the chosen protocol. Additionally, the zinc spinel is also prepared through an alternative route combining coprecipitation of oxalates from an aqueous solution with thermal decomposition under reflux conditions. The nanocrystalline powders are obtained as a pure crystalline phase already at the extremely low temperature of 75 °C and no further thermal treatment is needed. The structure and microstructure of the prepared materials is investigated by means of X-ray powder diffraction (XRPD), while X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analyses are used to gain information about the surface and bulk composition of the samples, respectively, confirming the expected stoichiometry. To investigate the effect of the synthesis protocol on the morphology of the obtained ferrites, transmission electron microscopy (TEM) observations are performed on selected samples. The magnetic properties of the cobalt and manganese spinels are also investigated using a superconducting quantum device magnetometer (SQUID) revealing hard and soft ferrimagnetic behavior, respectively.

Similar content being viewed by others
References
Dahl, J. A.; Maddux, B. L. S.; Hutchison, J. E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269.
Song, C. Fuel processing for low-temperature and high-temperature fuel cells. Challenges, and opportunities for sustainable development in the twenty-first century. Catal. Today 2002 77, 17–49.
Trost, B. M. On inventing reactions for atom economy. Acc. Chem. Res. 2002, 35, 695–705.
Herrmann, J. M.; Duchamp, C.; Karkmaz, M.; Hoai, B. T.; Lachheb, H.; Puzenat, E.; Guillard, C. Environmental green chemistry as defined by photocatalysis. J. Hazard. Mater. 2007, 146, 624–629.
Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd Ed.; Pergamon Press: India, 1998.
Cotton, F. A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th Ed.; John Wiley & Sons: New York, 1988.
Holleman, A. F.; Wieberg, E. Lehrbuch der Anorganischen Chemie, 101st Ed.; Walter de Gruyter: New York, 1995.
Kung, H. H. Transition Metal Oxides: Surface Chemistry and Catalysis. Elsevier: Amsterdam, 1989.
Rao, C. N. R.; Raveau, B. Transition Metal Oxides: Structure, Properties and Synthesis of Ceramic Oxides. Wiley VCH: Weinheim, 2009.
de Muro, I. G.; Insausti, M.; Lezama, L.; Rojo, T. Effects of the synthesis conditions on the magnetic and electrical properties of the BaFeO3−x oxide: Ametamagnetic behaviour. J. Solid State Chem. 2005, 178, 1712–1719.
Deng, Y.; Zhou, J. X.; Wu, D.; Du, Y. L.; Zhang, M. S.; Wang, D. H.; Yu, H. Q.; Tang, S. L.; Du, Y. W. Three-dimensional phases-connectivity and strong magnetoelectric response of self-assembled feather-like CoFe2O4-BaTiO3 nanostructures. Chem. Phys. Lett. 2010, 496, 301–305.
Rezlescu, N.; Doroftei, C.; Popa, P. D. Humidity-sensitive electrical resistivity of MgFe2O4 and Mg0.9Sn0.1Fe2O4 porous ceramics. Rom. J. Phys. 2007, 52, 353–360.
Florea, M.; Alifanti, M.; Parvulescu, V. I.; Mihaila-Tarabasanu, D.; Diamandescu, L.; Feder, M.; Negrila, C.; Frunza, L. Total oxidation of toluene on ferrite-type catalysts. Catal. Today 2009, 141, 361–366.
Scheffe, J. R.; Li, J.; Weimer, A. W. A spinel ferrite/hercynite water-splitting redox cycle. Int. J. Hydrogen Energ. 2010, 35, 3333–3340.
Menini, L.; Pereira, M. C.; Parreira, L. A.; Fabris, J. D.; Gusevskaya, E. V. Cobalt- and manganese-substituted ferrites as efficient single-site heterogeneous catalysts for aerobic oxidation of monoterpenic alkenes under solvent-free conditions. J. Catal. 2008, 254, 355–364.
Latham, A. H.; Williams, M. E. Controlling transport and chemical functionality of magnetic nanoparticles. Acc. Chem. Res. 2008, 41, 411–420.
Colombo, M.; Carregal-Romero, S.; Casula, M. F.; Gutierrez, L.; Morales, M. P.; Boehm, I. B.; Heverhagen, J. T.; Prosperi, D.; Parak, W. J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334.
Gijs, M. A. M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2010, 110, 1518–1563.
Nordhei, C.; Ramstad, A. L.; Nicholson, D. G. Nanophase cobalt, nickel and zinc ferrites: Synchrotron XAS study on the crystallite size dependence of metal distribution. Phys. Chem. Chem. Phys. 2008, 10, 1053–1066.
Costa, A. C. F. M.; Tortella, E.; Morelli, M. R.; Kiminami, R. H. G. A. Synthesis, microstructure and magnetic properties of Ni-Zn ferrites. J. Magn. Magn. Mater. 2003, 256, 174–182.
Thummer, K. P.; Chantbar, M. C.; Modi, K. B.; Baldha, G. J.; Joshi, H. H. Localized canted spin behaviour in ZnxMg1.5−x Mn0.5FeO4 spinel ferrite system. J. Magn. Magn. Mater. 2004, 280, 23–30.
Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents-Synthesis, Formation, Assembly and Applications; Springer: New York, 2009.
Schubert, U.; Hüsing, N. Synthesis of Inorganic Materials, 2nd Ed.; Wiley-VCH: Weinheim, 2005.
Bao, N. Z.; Shen, L. M.; Wang, Y. H.; Padhan, P.; Gupta, A. A facile thermolysis route to ferrite nanocrystals. J. Am. Chem. Soc. 2007, 129, 12374–12375.
Modeshia, D. R.; Walton, R. I. Solvothermal synthesis of perovskites and pyrochlores: Crystallization of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 39, 4303–4325.
Pinna, N.; Grancharov, S.; Beato, P.; Bonville, P.; Antonietti, M.; Niederberger, M. Magnetite nanocrystals: Nonaqueous synthesis, characterization, and solubility. Chem. Mater. 2005, 17, 3044–3049.
Grasset, F.; Labhsetwar, N.; Li, D.; Park, D. C.; Saito, N.; Haneda, H.; Cador, O.; Roisnel, T.; Mornet, S.; Duguet, E. et al. Synthesis and magnetic characterization of zinc ferrite nanoparticles with different environments: Powder, colloidal solution, and zinc ferrite-silica core-shell nanoparticles. Langmuir 2002, 18, 8209–8216.
Sun, S.; Zeng, H.; Robinson, D. B.; Raoux, S.; Rice, P. M.; Wang, S. X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279.
Baldi, G.; Bonacchi, D.; Lorenzi, G.; Innocenti, C.; Sangregorio, C. Cobalt ferrite nanoparticles: The control of the particle size and surface state and their effects on magnetic properties. J. Magn. Magn. Mater. 2007, 311, 10–16.
Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800.
Wu, J. H.; Ko, S. P.; Liu, H. L.; Kim, S.; Ju, J. S.; Kim, K. Y. Sub 5 nm magnetite nanoparticles: Synthesis, microstructure, and magnetic properties. Mater. Lett. 2007, 61, 3124–3129.
Rigby, E.; Kehr, W.; Meldrum, C. Preparation of coprecipitated NiZn ferrite. IEEE T. Magn. 1984, 20, 1506–1508.
Kanade, S. A.; Puri, V. Properties of thick film Ni0.6Co0.4 FeyMn2−y O4: (0⩾y⩾0.5) NTC ceramic. J. Alloys. Compd. 2009, 475, 352–355.
Gabal, M. A.; Al, A. Y. M. Effect of diamagnetic substitution on the structural, magnetic and electrical properties of NiFe2O4. Mater. Chem. Phys. 2009, 115, 578–584.
Toberer, E. S.; Joshi, A.; Seshadri, R. Template-free routes to macroporous monoliths of nickel and iron oxides: Toward porous metals and conformally coated pore walls. Chem. Mater. 2005, 17, 2142–2147.
Wang, M.; Ai, Z.; Zhang, L. Generalized preparation of porous nanocrystalline ZnFe2O4 superstructures from zinc ferrioxalate precursor and its superparamagnetic property. J. Phys. Chem. C 2008, 112, 13163–13170.
Diodati, S.; Nodari, L.; Natile, M. M.; Russo, U.; Tondello, E.; Lutterotti, L.; Gross, S. Highly crystalline strontium ferrites SrFeO3−δ : An easy and effective wet-chemistry synthesis. Dalton Trans. 2012, 41, 5517–5525.
Diodati, S.; Nodari, L.; Natile, M. M.; Caneschi, A.; de Julián Fernández, C.; Hoffmann, C.; Kaskel, S.; Lieb, A.; Di Noto, V.; Mascotto, S. et al. Coprecipitation of oxalates: An easy and reproducible wet-chemistry synthesis route for transition metal ferrites. Eur. J. Inorg. Chem. 2014, 875–887.
Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; Noyes Publications: Park Ridge, New Jersey, U.S.A, 2001.
Lobachev, A. N. Crystallization Processes Under Hydrothermal Conditions, 1st Ed.; Consultants Bureau: New York, 1973.
Zhou, J.; Ma, J. F.; Sun, C.; Xie, L. J.; Zhao, Z. Q.; Tian, H.; Wang, Y. G.; Tao, J. T.; Zhu, X. Y. Low-temperature synthesis of NiFe2O4 by a hydrothermal method. J. Am. Ceram. Soc. 2005, 88, 3535–3537.
Truong, Q. D.; Le, T. H.; Liu, J.-Y.; Chung, C.-C.; Ling, Y.-C. Synthesis of TiO2 nanoparticles using novel titanium oxalate complex towards visible light-driven photocatalytic reduction of CO2 to CH3OH. Appl. Catal. A-Gen. 2012, 437–438, 28–35.
Zhang, G. J.; Shen, Z. R.; Liu, M.; Guo, C. H.; Sun, P. C.; Yuan, Z. Y.; Li, B. H.; Ding, D. T.; Chen, T. H. Synthesis and characterization of mesoporous ceria with hierarchical nanoarchitecture controlled by amino acids. J. Phys. Chem. B 2006, 110, 25782–25790.
Truong, Q. D.; Kakihana, M. Hydrothermal growth of cross-linked hyperbranched copper dendrites using copper oxalate complex. J. Crystal Growth 2012, 348, 60–64.
Yang, J.; Mei, S.; Ferreira, J. M. F. Hydrothermal synthesis of nanosized titania powders: Influence of tetraalkyl ammonium hydroxides on particle characteristics. J. Am. Ceram. Soc. 2001, 84, 1696–1702.
Moulder, J. F.; Stickle, W. F.; Sobol, P. E.; Bomben, K. D. Handbook of X-ray Photoelectron Spectroscopy-A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corp.: Eden Prarie, Minnesota, 1992.
NIST XPS Database-Version 3.5. http://srdata.nist.gov/xps/.
Briggs, D.; Seah, M. P. Practical Surface Analysis: Volume 1-Auger and X-ray Photoelectron Spectroscopy, 2nd Ed.; John Wiley & Sons: New York, 1990.
Wang, Z. G.; Zu, X. T.; Zhu, S.; Wang, L. M. Green luminescence originates from surface defects in ZnO nanoparticles. Phys. E. 2006, 35, 199–202.
Shirley, D. A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4713.
High Tech International Services Snc XPS_AES Program v. 4.7; www.htis.it, 2006.
Kwok, R. H. W. XPSPEAK, 4.1; University of Hong Kong: Hong Kong, 1994.
Lutterotti, L. MAUD Program; Università degli Studi di Trento: Trento, 1998.
Aono, H.; Hirazawa, H.; Naohara, T.; Maehara, T. Surface study of fine MgFe2O4 ferrite powder prepared by chemical methods. Appl. Surf. Sci. 2008, 254, 2319–2324.
Bayoumi, W. Structural and electrical properties of zinc-substituted cobalt ferrite. J. Mater. Sci. 2007, 42, 8254–8261.
Kakihana, M.; Yoshimura, M. Synthesis and characteristics of complex multicomponent oxides prepared by polymer complex method. Bull. Chem. Soc. Jpn. 1999, 72, 1427–1443.
Lessing, P. A. Mixed-cation oxide powders via polymeric precursors. Am. Ceram. Soc. Bull. 1989, 68, 1002–1007.
Segal, D. Chemical synthesis of ceramic materials. J. Mater. Chem. 1997, 7, 1297–1305.
Jiang, Q.-H.; Nan, C.-W.; Shen, Z.-J. Synthesis and properties of multiferroic La-modified BiFeO3 ceramics. J. Am. Ceram. Soc. 2006, 89, 2123–2127.
Haetge, J.; Suchomski, C.; Brezesinski, T. Ordered mesoporous MFe2O4 (M = Co, Cu, Mg, Ni, Zn) thin films with nanocrystalline walls, uniform 16 nm diameter pores and high thermal stability: Template-directed synthesis and characterization of redox active trevorite. Inorg. Chem. 2010, 49, 11619–11626.
Broadbent, D.; Dollimore, D.; Dollimore, J. The thermal decomposition of oxalates. Part V. The thermal decomposition of nickel oxalate dihydrate. J. Chem. Soc. A 1966, 278–281.
Broadbent, D.; Dollimore, D.; Dollimore, J. The thermal decomposition of oxalates. Part IX. The thermal decomposition of the oxalate complexes of iron. J. Chem. Soc. A 1967, 451–454.
Dollimore, D.; Griffiths, D. L.; Nicholson, D. The thermal decomposition of oxalates. Part II. Thermogravimetric analysis of various oxalates in air and in nitrogen. J. Chem. Soc. 1963, 2617–2623.
Dollimore, D.; Nicholson, D. The thermal decomposition of oxalates. Part VI. The decomposition and surface properties of ferric oxalate. J. Chem. Soc. A 1966, 281–284.
Dollimore, D.; Nicholson, D. The thermal decomposition of oxalates. Part I. The variation of surface area with the temperature of treatment in air. J. Chem. Soc. 1962, 960–965.
Gull, S. F.; Daniell, G. J. Image reconstruction from incomplete and noisy data. Nature 1978, 272, 686–690.
Wychoff, R. W. G. Structure of Crystals; The Chemical Catalogue Company Inc.: New York, 1931.
Passerini, L. Ricerche sugli spinelli. II. I composti: CuAl2O4; MgAl2O4; MgFe2O4; ZnAl2O4; ZnCr2O4; ZnFe2O4; MnFe2O4. Gazz. Chim. Ital. 1930, 60, 389–399.
Montoro, V. Miscibilità tra gli ossidi di ferro e di manganese. Gazz. Chim. Ital. 1938, 68, 728–733.
Kremnović, A.; Antić, B.; Vuĉinić-Vasić, M.; Colomban, P.; Jovalekić, C.; Bibić, N.; Kahlenberg, V.; Leoni, M. Temperature-induced structure and microstructure evolution of nanostructured Ni0.9Zn0.1O. J. Appl. Cryst. 2010, 43, 699–709.
Crystallography Open Database. http://www.crystallography. net/.
Le Bail, A. In Proceedings of the International Conference on Accuracy in powder Diffraction ll. NIST, Gaithersburg, 1992, pp142–153.
Ceccone, G.; Marmorato, P.; Ponti, J.; Rossi, F.; Kaulich, B.; Gianocelli, A.; Pascolo, L.; Salome, M.; Kiskinova, M. Synchrotron radiation X-ray fluorescence mapping of cobalt ferrite nanoparticles in Balb/3T3 fibroblast cells. Pacifichem 2010-International Chemical Congress of Pacific Basin Societies, Honolulu, U.S.A., 2010.
Schrader, B. Infrared and Raman Spectroscopy: Methods and Applications, 1st Ed.; VCH: Weinheim, Germany, 1995.
Ayyappan, S.; Mahadevan, S.; Chandramohan, P.; Srinivasan, M. P.; Philip, J.; Raj, B. Influence of Co2+ ion concentration on the size, magnetic properties, and purity of CoFe2O4 spinel ferrite nanoparticles. J. Phys. Chem. C 2010, 114, 6334–6341.
Chandramohan, P.; Srinivasan, M. P.; Velmurugan, S.; Narasimhan, S. V. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96.
Varshney, D.; Verma, K.; Kumar, A. Structural and vibrational properties of ZnxMn1−x Fe2O4 (x = 0.0, 0.25, 0.50, 0.75, 1.0) mixed ferrites. Mater. Chem. Phys. 2011, 131, 413–419.
Ahlawat, A.; Sathe, V. G.; Reddy, V. R.; Gupta, A. Mossbauer, Raman and X-ray diffraction studies of superparamagnetic NiFe2O4 nanoparticles prepared by sol-gel auto-combustion method. J. Magn. Magn. Mater. 2011, 323, 2049–2054.
Lazarević, Z. Ž.; Jovalekić, Č.; Milutinović, A.; Romcević, M. J.; Romcević, N. Ž. Preparation and characterization of nano ferrites. Acta Phys. Pol. A 2012, 121, 682–686.
Wang, Z. W.; Schiferl, D.; Zhao, Y. S.; O’Neill, H. S. C. High pressure Raman spectroscopy of spinel-type ferrite ZnFe2O4. J. Phys. Chem. Solids 2003, 64, 2517–2523.
Nongjai, R.; Khan, S.; Asokan, K.; Ahmed, H.; Khan, I. Magnetic and electrical properties of In doped cobalt ferrite nanoparticles. J. Appl. Phys. 2012, 112, 084321.
Yamashita, O.; Ikeda, T. Effect of polishing stress on Raman spectra of the Mn-Zn ferrite. J. Appl. Phys. 2004, 95, 1743–1748.
Lee, H.; Jung, J. C.; Kim, H.; Chung, Y.-M.; Kim, T. J.; Lee, S. J.; Oh, S.-H.; Kim, Y. S.; Song, I. K. Effect of divalent metal component (MeII) on the catalytic performance of MeIIFe2O4 catalysts in the oxidative dehydrogenation of n-butene to 1,3-butadiene. Catal. Lett. 2008, 124, 364–368.
Zhang, S. X.; Niu, H. Y.; Cai, Y. Q.; Zhao, X. L.; Shi, Y. L. Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem. Eng. J. 2010, 158, 599–607.
Domenichini, B.; Pataut, G.; Bourgeois, S. Stabilization of polar solid oxide surfaces: Competition between adsorption and reconstruction. Surf. Interface Anal. 2002, 34, 540–544.
Bera, S.; Prince, A. A. M.; Velmurugan, S.; Raghavan, P. S.; Gopalan, R.; Panneerselvam, G.; Narasimhan, S. V. Formation of zinc ferrite by solid-state reaction and its characterization by XRD and XPS. J. Mater. Sci. 2001, 36, 5379–5384.
Herranz, T.; Rojas, S.; Ojeda, M.; Pérez-Alonso, F. J.; Terreros, P.; Pirota, K.; Fierro, J. L. G. Synthesis, structural features, and reactivity of Fe-Mn mixed oxides prepared by microemulsion. Chem. Mater. 2006, 18, 2364–2375.
Mittal, V. K.; Bera, S.; Nithya, R.; Srinivasan, M. P.; Velmurugan, S.; Narasimhan, S. V. Solid state synthesis of Mg-Ni ferrite and characterization by XRD and XPS. J. Nucl. Mater. 2004, 335, 302–310.
Mittal, V. K.; Chandramohan, P.; Bera, S.; Srinivasan, M. P.; Velmurugan, S.; Narasimhan, S. V. Cation distribution in NixMg1−x Fe2O4 studied by XPS and Mössbauer spectroscopy. Solid State Commun. 2006, 137, 6–10.
Baruwati, B.; Rana, R. K.; Manorama, S. V. Further insights in the conductivity behavior of nanocrystalline NiFe2O4. J. Appl. Phys. 2007, 101, 014302.
Nawale, A. B.; Kanhe, N. S.; Patil, K. R.; Bhoraskar, S. V.; Mathe, V. L.; Das, A. K. Magnetic properties of thermal plasma synthesized nanocrystalline nickel ferrite (NiFe2O4). J. Alloys. Compd. 2011, 509, 4404–4413.
Glisenti, A.; Natile, M. M.; Galenda, A. PrMnO3 prepared by the citrate gel method, studied by XPS. Surf Sci. Spec. 2009, 16, 67–74.
Murray, J. W.; Dillard, J. G.; Giovanoli, R.; Moers, H.; Stumm, W. Oxidation of manganese (II): Initial mineralogy, oxidation state and ageing. Geochim. Cosmochim. Ac. 1985, 49, 463–470.
Gupta, R. P.; Sen, S. K. Calculation of multiplet structure of core p-vacancy levels. Phys. Rev. B 1974, 10, 71–77.
Kim, K. J.; Lee, H. J.; Park, J. Y. Cationic behavior and the related magnetic and magnetotransport properties of manganese ferrite thin films. J. Magn. Magn. Mater. 2009, 321, 3706–3711.
Hu, J.; Lo, I. M. C.; Chen, G. H. Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 2005, 21, 11173–11179.
Natile, M. M.; Glisenti, A. Surface reactivity of NiO: Interaction with methanol. Chem. Mater. 2002, 14, 4895–4903.
Bardhan, A.; Ghosh, C. K.; Mitra, M. K.; Das, G. C.; Mukherjee, S.; Chattopadhyay, K. K. Low temperature synthesis of zinc ferrite nanoparticles. Solid State Sci. 2010, 12, 839–844.
Tahir, A. A.; Wijayantha, K. G. U. Photoelectrochemical water splitting at nanostructured ZnFe2O4 electrodes. J. Photoch. Photobio. A 2010, 216, 119–125.
Dionne, G. F. Magnetic Oxides, 1st Ed.; Springer: London, 2009.
Tilley, R.J. D. Understanding Solids-The Science of Materials; J. Wiley & Sons: Chichester, West Sussex, England, 2004.
Coey, J. M. D. Magnetism and Magnetic Materials, 1st Ed.; Cambridge University Press: New York, 2010.
Özgür, Ü.; Alivov, Y.; Morkoç, H. Microwave ferrites, part 1: Fundamental properties. J. Mater. Sci.: Mater. Electron. 2009, 20, 789–834.
Lu, H. M.; Zheng, W. T.; Jiang, Q. Saturation magnetization of ferromagnetic and ferrimagnetic nanocrystals at room temperature. J. Phys. D: Appl. Phys. 2007, 40, 320–325.
Linderoth, S.; Hendriksen, P. V.; Bødker, F.; Wells, S.; Davies, K.; Charles, S. W.; Mørup, S. On spin-canting in maghemite particles. J. Appl. Phys. 1994, 75, 6583–6585.
Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D: Appl. Phys. 2002, 35, R15–R42.
Peddis, D.; Yaacoub, N.; Ferretti, M.; Martinelli, A.; Piccaluga, G.; Musinu, A.; Cannas, C.; Navarra, G.; Greneche, J. M.; Fiorani, D. Cationic distribution and spin canting in CoFe2O4 nanoparticles. J. Phys.: Condens. Matter 2011, 23, 426004.
Kim, B. H.; Lee, N.; Kim, H.; An, K.; Park, Y. I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S. G.; Na, H. B. et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631.
Sun, Q.-C.; Birkel, C. S.; Cao, J. B.; Tremel, W.; Musfeldt, J. L. Spectroscopic signature of the superparamagnetic transition and surface spin disorder in CoFe2O4 nanoparticles. ACS Nano 2012, 6, 4876–4883.
Zhang, Y.; Liu, Y.; Fei, C. L.; Yang, Z.; Lu, Z. H.; Xiong, R.; Yin, D.; Shi, J. The temperature dependence of magnetic properties for cobalt ferrite nanoparticles by the hydrothermal method. J. Appl. Phys. 2010, 108, 084312.
Zhao, L. J.; Zhang, H. J.; Xing, Y.; Song, S. Y.; Yu, S. Y.; Shi, W. D.; Guo, X. M.; Yang, J. H.; Lei, Y. Q.; Cao, F. Studies on the magnetism of cobalt ferrite nanocrystals synthesized by hydrothermal method. J. Solid State Chem. 2008, 181, 245–252.
Fan, G. L.; Gu, Z. J.; Yang, L.; Li, F. Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 2009, 155, 534–541.
Chen, L. Y.; Shen, Y. M.; Bai, J. F. Large-scale synthesis of uniform spinel ferrite nanoparticles from hydrothermal decomposition of trinuclear heterometallic oxo-centered acetate clusters. Mater. Lett. 2009, 63, 1099–1101.
Ren, G.-H.; Yu, Z.-S. Synthesis of monodisperse Fe3O4 and MnFe2O4 nanospheres by using a solvothermal reduction method. Solid State Phenom. 2012, 181-182, 393–396.
Holden, A.; Singer, P. Crystals and Crystal Growing; Anchor Books Doubleday & Company Inc.: Garden City, New York, 1971.
Chen, X. L.; Fan, H. Q.; Liu, L. J. Synthesis and crystallization behavior of lead titanate from oxide precursors by a hydrothermal route. J. Cryst. Growth 2005, 284, 434–439.
MacLaren, I.; Ponton, C. B. A TEM and HREM study of particle formation during barium titanate synthesis in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 1267–1275.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Diodati, S., Pandolfo, L., Caneschi, A. et al. Green and low temperature synthesis of nanocrystalline transition metal ferrites by simple wet chemistry routes. Nano Res. 7, 1027–1042 (2014). https://doi.org/10.1007/s12274-014-0466-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0466-3