Abstract
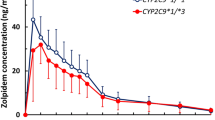
Zolpidem is indicated for the short-term treatment of insomnia and it is predominantly metabolized by CYP3A4, and to a lesser extent by CYP2C19, CYP1A2, and CYP2C9. Therefore, we evaluated the effects of CYP2C19 genetic polymorphisms on the pharmacokinetics of zolpidem in healthy male subjects. Thirty-two male subjects were recruited and all subjects were classified into three groups according to their genotypes: CYP2C19EM (CYP2C19*1/*1, n = 12), CYP2C19IM (CYP2C19*1/*2 or *1/*3, n = 10), and CYP2C19PM (CYP2C19*2/*2, *2/*3 or *3/*3, n = 10). The pharmacokinetic parameters of zolpidem were compared in three CYP2C19 genotype groups after zolpidem administration with or without a CYP3A4 inhibitor at steady-state concentration. Plasma concentrations of zolpidem were determined up to 12 h after drug administration by liquid chromatography-tandem mass spectrometry method. The maximum plasma concentration (Cmax) differed, but mean total area under the plasma concentration–time curve (AUCinf), half-life (t1/2), and apparent oral clearance (CL/F) of zolpidem administered alone did not significantly differ among the three different CYP2C19 genotype groups. Furthermore, when zolpidem was administered with a CYP3A4 inhibitor at steady-state concentration, there were no significant differences in any of the pharmacokinetic parameters of zolpidem in relation to CYP2C19 genotypes. In conclusion, we did not find any evidence for the impact of CYP2C19 genetic polymorphisms on the pharmacokinetic parameters of zolpidem.

Similar content being viewed by others
References
AbbVie Inc. Biaxin (clarithromycin) prescribing information (issued Jul 2014). http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050662Orig1s055,050698Orig1s035,050775Orig1s023lbl.pdf. Accessed 1 Dec 2017
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71:550–555
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011b) Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32:1303–1308
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50:683–689
Byeon JY, Kim YH, Na HS, Jang JH, Kim SH, Lee YJ, Bae JW, Kim IS, Jang CG, Chung MW, Lee SY (2015a) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38:2083–2091
Byeon JY, Lee HI, Lee YJ, Lee JE, Kim SH, Kim YH, Na HS, Jang CG, Lee SY (2015b) Determination of zolpidem in human plasma by liquid chromatography-tandem mass spectrometry for clinical application. J Chromatogr B 986–987:129–134
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109:476–480
Choi CI, Bae JW, Jang CG, Lee SY (2012a) Tamsulosin exposure is significantly increased by the CYP2D6*10/*10 genotype. J Clin Pharmacol 52:1934–1938
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012b) CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68:149–154
Darcourt G, Pringuey D, Sallière D, Lavoisy J (1999) The safety and tolerability of zolpidem–an update. J Psychopharmacol 13:81–93
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994a) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
De Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994b) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41:913–958
Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA Jr, O’Mara EM, Hall SD (1998) The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther 64:133–143
Greenblatt DJ, Roth T (2012) Zolpidem for insomnia. Expert Opin Pharmacother 13:879–893
Greenblatt DJ, von Moltke LL, Harmatz JS, Counihan M, Graf JA, Durol AL, Mertzanis P, Duan SX, Wright CE, Shader RI (1998) Inhibition of triazolam clearance by macrolide antimicrobial agents: in vitro correlates and dynamic consequences. Clin Pharmacol Ther 64:278–285
Hoehns J, Perry P (1993) Zolpidem: a nonbenzodiazepine hypnotic for treatment of insomnia. Clin Pharm 12:814–828
Holm KJ, Goa KL (2000) Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 59:865–889
Inagaki T, Miyaoka T, Tsuji S, Inami Y, Nishida A, Horiguchi J (2010) Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry 12:e1–e8
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C (2007) Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116:496–526
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40:382–390
Kim MJ, Byeon JY, Kim YH, Kim SH, Lee CM, Jung EH, Chae WK, Lee YJ, Jang CG, Lee SY, Choi CI (2018) Effect of the CYP2D6*10 allele on the pharmacokinetics of clomiphene and its active metabolites. Arch Pharm Res 41:347–353
Langer SZ, Arbilla S (1988) Imidazopyridines as a tool for the characterization of benzodiazepine receptors: a proposal for a pharmacological classification as omega receptor subtypes. Pharmacol Biochem Behav 29:763–766
Langtry HD, Benfield P (1990) Zolpidem. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs 40:291–313
Lee HI, Bae JW, Choi CI, Lee YJ, Byeon JY, Jang CG, Lee SY (2014) Strongly increased exposure of meloxicam in CYP2C9*3/*3 individuals. Pharmacogenet Genomics 24:113–117
Lee HJ, Kim YH, Kim SH, Lee CM, Yang AY, Jang CG, Lee SY, Bae JW, Choi CI (2016) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch Pharm Res 39:1013–1019
Perrault G, Morel E, Sanger DJ, Zivkovic B (1990) Differences in pharmacological profiles of a new generation of benzodiazepine and non-benzodiazepine hypnotics. Eur J Pharmacol 187:487–494
Pichard L, Gillet G, Bonfils C, Domergue J, Thénot JP, Maurel P (1995) Oxidative metabolism of zolpidem by human liver cytochrome P450S. Drug Metab Dispos 23:1253–1262
Sanger DJ, Depoortere H (1998) The pharmacology and mechanism of action of zolpidem. CNS Drug Rev 4:323–340
Shen M, Shi Y, Xiang P (2013) CYP3A4 and CYP2C19 genetic polymorphisms and zolpidem metabolism in the Chinese Han population: a pilot study. Forensic Sci Int 227:77–81
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Swainston Harrison T, Keating GM (2005) Zolpidem: a review of its use in the management of insomnia. CNS Drugs 19:65–89
Von Moltke LL, Greenblatt DJ, Granda BW, Duan SX, Grassi JM, Venkatakrishnan K, Harmatz JS, Shader RI (1999) Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations. Br J Clin Pharmacol 48:89–97
Zhou SF, Liu JP, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41:89–295
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (NRF-2016R1A2B4007381).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Byeon, JY., Kim, YH., Kim, SH. et al. Effects of genetic polymorphisms of CYP2C19 on the pharmacokinetics of zolpidem. Arch. Pharm. Res. 41, 861–866 (2018). https://doi.org/10.1007/s12272-018-1065-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1065-8