Abstract
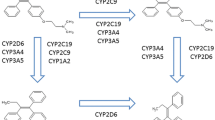
Clomiphene citrate, a selective estrogen receptor modulator, is metabolized into its 4-hydroxylated active metabolites, primarily by CYP2D6. In this study, we investigated the effects of the most common CYP2D6 variant allele in Asians, CYP2D6*10, on the pharmacokinetics of clomiphene and its two active metabolites (4-OH-CLO and 4-OH-DE-CLO) in healthy Korean subjects. A single 50-mg oral dose of clomiphene citrate was given to 22 Korean subjects divided into three genotype groups according to CYP2D6 genotypes, CYP2D6*wt/*wt (n = 8; *wt = *1 or *2), CYP2D6*wt/*10 (n = 8) and CYP2D6*10/*10 (n = 6). Concentrations of clomiphene and its metabolites were determined using a validated HPLC–MS/MS analytical method in plasma samples collected up to 168 h after the drug intake. There was a significant difference only in the Cmax of clomiphene between three CYP2D6 genotype groups (p < 0.05). Paradoxically, the elimination half-life (t1/2) and AUC of both active metabolites were all significantly increased in the CYP2D6*10 homozygous carriers, compared with other genotype groups (all p < 0.001). The AUCinf of corrected clomiphene active moiety in CYP2D6*10/*10 subjects was 2.95- and 2.05-fold higher than that of CYP2D6*wt/*wt and *wt/*10 genotype groups, respectively (both p < 0.001). Along with the partial impacts on the biotransformation of clomiphene and its metabolites by CYP2D6 genetic polymorphism, further studies on the effects of other CYP enzymes in a multiple-dosing condition can provide more definite evidence for the inter-individual variabilities in clomiphene pharmacokinetics and/or drug response.


Similar content being viewed by others
References
Byeon JY, Kim YH, Na HS, Jang JH, Kim SH, Lee YJ, Bae JW, Kim IS, Jang CG, Chung MW, Lee SY (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38:2083–2091
Chaudhry SR, Muhammad S, Eidens M, Klemm M, Khan D, Efferth T, Weisshaar MP (2014) Pharmacogenetic prediction of individual variability in drug response based on CYP2D6, CYP2C9 and CYP2C19 genetic polymorphisms. Curr Drug Metab 15(7):711–718
CLOMID label information (2012) Sanofi-Aventis U.S. LLC, Bridgewater, NJ, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf. Accessed 27 Oct 2017
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310:1062–1075
Dickey RP (1996) Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update 2:483–506
Ghobadi C, Gregory A, Crewe HK, Rostami-Hodjegan A, Lennard MS (2008) CYP2D6 is primarily responsible for the metabolism of clomiphene. Drug Metab Pharmacokinet 23:101–105
Ghobadi C, Mirhosseini N, Shiran MR, Moghadamnia A, Lennard MS, Ledger WL, Rostami-Hodjegan A (2009) Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J Clin Pharmacol 49:147–154
Hirota T, Eguchi S, Ieiri I (2013) Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab Pharmacokinet 28(1):28–37
Homburg R (2005) Clomiphene citrate—end of an era? A mini-review. Hum Reprod 20:2043–2051
Ji M, Kim KR, Lee W, Choe W, Chun S, Min WK (2016) Genetic polymorphism of CYP2D6 and clomiphene concentrations in infertile patients with ovulatory dysfunction treated with clomiphene citrate. J Korean Med Sci 31:310–314
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40(3):382–390
Lee HJ, Kim YH, Kim SH, Lee CM, Yang AY, Jang CG, Lee SY, Bae JW, Choi CI (2016) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch Pharm Res 39(7):1013–1019
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Lim HS, Ju Lee H, Seok Lee K, Sook Lee E, Jang IJ, Ro J (2007) Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 25:3837–3845
Lundqvist E, Johansson I, Ingelman-Sundberg M (1999) Genetic mechanisms for duplication and multiduplication of the human CYP2D6 gene and methods for detection of duplicated CYP2D6 genes. Gene 226:327–338
Ma JD, Lee KC, Kuo GM (2012) Clinical application of pharmacogenomics. J Pharm Pract 25(4):417–427
Mazzarino M, Biava M, de la Torre X, Fiacco I, Botrè F (2013) Characterization of the biotransformation pathways of clomiphene, tamoxifen and toremifene as assessed by LC-MS/(MS) following in vitro and excretion studies. Anal Bioanal Chem 405:5467–5487
Mürdter TE, Kerb R, Turpeinen M, Schroth W, Ganchev B, Böhmer GM, Igel S, Schaeffeler E, Zanger U, Brauch H, Schwab M (2012) Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum Mol Genet 21:1145–1154
Naveen AT, Adithan C, Soya SS, Gerard N, Krishnamoorthy R (2006) CYP2D6 genetic polymorphism in South Indian populations. Biol Pharm Bull 29:1655–1658
PharmVar (2017) CYP allele nomenclature. http://www.pharmvar.org/genes. Accessed 30 Dec 2017
Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S (2013) Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 8(12):e82562
Probst-Schendzielorz K, Viviani R, Stingl JC (2015) Effect of Cytochrome P450 polymorphism on the action and metabolism of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol 11(8):1219–1232
Rostami-Hodjegan A, Lennard MS, Tucker GT, Ledger WL (2004) Monitoring plasma concentrations to individualize treatment with clomiphene citrate. Fertil Steril 81:1187–1193
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2016R1A2B4007381).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, MJ., Byeon, JY., Kim, YH. et al. Effect of the CYP2D6*10 allele on the pharmacokinetics of clomiphene and its active metabolites. Arch. Pharm. Res. 41, 347–353 (2018). https://doi.org/10.1007/s12272-018-1005-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1005-7