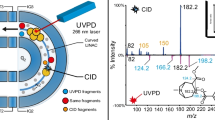
Abstract
Over the past decades, mass spectrometry technologies have been developed to obtain mass accuracies of one ppm or less. Of the newly developed technologies, quadrupole time-of–flight mass spectrometry (Q-TOF–MS) has emerged as being well suited to routine and high-throughput analyses of pharmaceuticals. Dietary supplements and functional foods have frequently been found to be contaminated with pharmaceuticals. In our continuous efforts to develop methodologies to protect public health against adulterated dietary supplements, we have constructed a mass spectral database for 21 H1-antihistamines encountered as adulterants by using liquid chromatography-electrospray ionization (LC-ESI)/Q-TOF–MS, and have proposed their possible collision-induced dissociation pathways. This database will be very useful for the rapid and accurate detection of H1-antihistamines (known) and their analogues (unknown) illegally added to dietary supplements as well as in other sample matrices.








Similar content being viewed by others
References
Arayne MS, Sultana N, Mirza AZ, Siddiqui FA (2010) Simultaneous determination of gliquidone, fexofenadine, buclizine, and levocetirizine in dosage formulation and human serum by RP-HPLC. J Chromatogr Sci 48:382–385
Cataldi M, Borriello F, Granata F, Annunziato L, Marone G (2014) Histamine receptors and antihistamines: from discovery to clinical applications. Chem Immunol Allergy 100:214–226
Chai Y, Wang L, Wang L (2016) How does a C=C double bond cleave in the gas phase? Fragmentation of protonated ketotifen in mass spectrometry. J Mass Spectrom 51:1105–1110
Chan TYK (2009) Outbreaks of severe hypoglycaemia due to illegal sexual enhancement products containing undeclared glibenclamide. Pharmacoepidemiol Drug Saf 18:1250–1251
Chen G, Daaro I, Pramanik BN, Piwinski JJ (2009) Structural characterization of in vitro rat liver microsomal metabolites of antihistamine desloratadine using LTQ-Orbitrap hybrid mass spectrometer in combination with online hydrogen/deuterium exchange HR-LC/MS. J Mass Spectrom 44:203–213
Ferrer I, Heine CE, Thurman EM (2004) Combination of LC/TOF-MS and LC/Ion trap MS/MS for the identification of diphenhydramine in sediment samples. Anal Chem 76:1437–1444
Fujimaki K, Lee X-P, Kumazawa T, Sato J, Sato K (2006) Determination of some antiallergic drugs in human plasma by direct-injection high-performance liquid chromatography-tandem mass spectrometry. Forensic Toxicol 24:8–16
Gergov M, Robson JN, Ojanperä I, Heinonen OP, Vuori E (2001) Simultaneous screening and quantitation of 18 antihistamine drugs in blood by liquid chromatography ionspray tandem mass spectrometry. Forensic Sci Int 121:108–115
Gomes PJ (2014) Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol 14:451–456
Hanrahan LP, Paramore LC (2003) Aeroallergens, allergic rhinitis, and sedating antihistamines: risk factors for traumatic occupational injury and economic impact. Am J Ind Med 44:438–446
Hasegawa C, Kumazawa T, Lee X-P, Fujishiro M, Kuriki A, Marumo A, Seno H, Sato K (2006) Simultaneous determination of ten antihistamine drugs in human plasma using pipette tip solid-phase extraction and gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20:537–543
Howard ME, Desai AV, Grunstein RR, Hukins C, Armstrong JG, Joffe D, Swann P, Campbell DA, Pierce RJ (2004) Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med 170:1014–1021
Jackson G, Arver S, Banks I, Stecher VJ (2010) Counterfeit phosphodiesterase type 5 inhibitors pose significant safety risks. Int J Clin Pract 64:497–504
Kageura M, Kataoka M, Hara K, Nagata T (1986) Changed compounds of hydroxyzine in human urine. Forensic Sci Int 32:229–235
Kajita J, Inano K, Fuse E, Kuwabara T, Kobayashi H (2002) Effects of olopatadine, a new antiallergic agent, on human liver microsomal cytochrome P450 activities. Drug Metab Dispos 30:1504–1511
Kim JY, Do J-A, Choi JY, Cho S, Kim W-S, Yoon C-Y (2015a) Development and validation of an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihihistaminics in dietary supplements. Biomed Chromatogr 29:465–474
Kim JY, Choi JY, Yoon CY, Cho S, Kim WS, Do JA (2015b) LC-MS/MS monitoring of 22 illegal antihistamine compounds in health food products from the Korean market. J Korean Soc Appl Biol Chem 58:137–147
Kumar L, Alam MdS, Meena CL, Jain R, Bansal AK (2009) Fexofenadine hydrochloride. In: Brittain HG (ed) Profiles of drug substances, excipients and related methodology 34. Elsevier, Cambridge, pp 150–192
Lee JH, Kim HJ, Mandava S, Hwang J, Park HJ, Cho S, Baek SY, Lee J (2015) Identification of a new tadalafil analogue in an adulterated dietary supplement: trans-Bisprehomotadalafil. J Pharm Biomed Anal 115:352–358
Lee JH, Mandava S, Baek SY, Lee YM (2016a) Identification and structural elucidation of three new tadalafil analogues found in a dietary supplement. J Pharm Biomed Anal 123:1–9
Lee JH, Park HN, Ganganna B, Jeong JH, Park SK, Lee J, Baek SY (2016b) Isolation and structural elucidation of a new tadalafil analogue in health supplements: bisprenortadalafil. Food Addit Contam Part A 33:945–952
Low MY, Zeng Y, Li L, Ge XW, Lee R, Bloodworth BC, Koh HL (2009) Safety and quality assessment of 175 illegal sexual enhancement products seized in red-light districts in Singapore. Drug Saf 32:1141–1146
McClure RA, Chumbley CW, Reyzer ML, Wilson K, Caprioli RM, Gore JC, Pham W (2013) Identification of promethazine as an amyloid-binding molecule using a fluorescence high-throughput assay and MALDI imaging mass spectrometry. Neuroimage Clin 2:620–629
Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR Jr (1990) Torsades de pointes occurring in association with terfenadine use. JAMA 264:2788–2790
Moreno RA, Olivera-Silva D, Sverdloff CE, Borges BC, Galvinas PAR, Astigarraga RB, Borges NC (2009) Determination of Chlorpheniramine in human plasma by HPLC-ESI-MS/MS: application to a dexchlorpheniramine comparative bioavailability study. Biomed Chromatogr 24:774–781
Niessen WMA (2011) Fragmentation of toxicologically relevant drugs in positive-ion liquid chromatography tandem mass spectrometry. Mass Spectrom Rev 30:626–663
Olasińska-Wiśniweska A, Olasiński J, Grajek S (2014) Cardiovascular safety of antihistamines. Postepy Dermatol Alergol 31:182–186
Patel DN, Low W-L, Tan LL, Tan M-MB, Zhang Q, Low M-Y, Chan C-L, Koh H-L (2012) Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigilance database from 1998 to 2009. Clin Toxicol 50:481–489
Picard N, Dridi D, Sauvage F-L, Boughattas NA, Marquet P (2009) General unknown screening procedure for the characterization of human drug metabolites: application to loratadine phase I metabolism. J Sep Sci 32:2209–2217
Poluzzi E, Raschi E, Godman B, Koci A, Moretti U, Kalaba M, Wettermark B, Sturkenboom M, De Ponti F (2015) Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe. PLoS ONE 10:e0119551. doi:10.1371/journal.pone.0119551
Poon WT, Lam YH, Lee HHC, Ching CK, Chan WT, Chan SS, Lai CK, Tse ML, Chan AYW, Mak TWL (2009) Outbreak of hypoglycaemia: sexual enhancement products containing oral hypoglycaemic agent. Hong Kong Med J 15:196–200
Scadding GK (2015) Optimal management of allergic rhinitis. Arch Dis Child 100:576–582
Shakya AK, Arafat TA, Abuawwad AN, Melhim M, Al-Ghani J, Yacoub MJ (2009) Simultaneous determination of triprolidine and pseudoephedrine in human plasma by liquid chromatography-ion trap mass spectrometry. J Chromatogr B 877:4071–4078
Sharma M, Bennett C, Cohen SN, Carter B (2014) H1-antihistamines for chronic spontaneous urticaria. Cochrane Database Syst Rev CD006137:1–252
Sher N, Siddiqui FA, Hasan N, Shafi N, Zubair A, Mirza Z (2014) Simultaneous determination of antihistamine anti-allergic drugs, cetirizine, domperidone, chlorphenamine maleate, loratadine, meclizine and buclizine in pharmaceutical formulations, human serum and pharmacokinetics application. Anal Methods 6:2704–2714
Simons FER (1999) Non-cardiac adverse effects of antihistamines (H1-receptor antagonists). Clin Exp Allergy 29(Suppl 3):116–124
Simons FER, Simons KJ (2011) Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol 128:1139–1150
Szelenyi I (2014) Allergic rhinitis: meaningful and less meaningful combination treatments including reminiscences. Pharmazie 69:414–416
Tevell A, Bondesson U, Törneke K, Hedeland M (2004) Identification of some new clemastine metabolites in dog, horse, and human urine with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 18:2267–2272
Yanai K, Rogala B, Chugh K, Paraskakis E, Pampura AN, Boev R (2012) Safety considerations in the management of allergic diseases: focus on antihistamines. Curr Med Res Opin 28:623–642
Yang L, Clement RP, Kantesaria B, Reyderman L, Beaudry F, Grandmaison C, Di Donato L, Masse R, Rudewicz PJ (2003) Validation of a sensitive and automated 96-well solid-phase extraction liquid chromatography–tandem mass spectrometry method for the determination of desloratadine and 3-hydroxydesloratadine in human plasma. J Chromatogr B 792:229–240
Acknowledgements
This research was supported by a Grant (13181MFDS724) from the Ministry of Food and Drug Safety in Korea. We thank the Central Laboratory of Kangwon National University for providing us with technical assistance on the spectroscopic experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jung-Ah Do and Eunyoung Noh have been contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Do, JA., Noh, E., Yoon, SB. et al. Collision-induced dissociation pathways of H1-antihistamines by electrospray ionization quadrupole time-of-flight mass spectrometry. Arch. Pharm. Res. 40, 736–745 (2017). https://doi.org/10.1007/s12272-017-0921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0921-2