Abstract
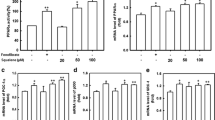
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that regulate the expression of multiple genes involved in metabolic, anti-inflammatory, and developmental processes. This study evaluated the PPARs transactivational effects of thirteen cembranoid diterpenoids 1–13 from the soft coral Lobophytum crassum, using PPAR-responsive elements–luciferase reporter and GAL4–PPAR chimera assays. All isolated compounds activated the transcription of PPARs in a dose-dependent manner, with EC50 values ranging from 2.07 ± 1.73 to 130.20 ± 1.85 μM. Moreover, compounds 6–9 affected the transactivation of all three PPAR types, PPARα, γ, β(δ), in a dose-dependent manner, with EC50 values in a ranging from 11.92 ± 1.23 to 122.50 ± 2.12 μM. These results provide a scientific rationale for further studies on the soft coral L. crassum and its diterpenoid constituents to develop medicinal products against inflammatory and metabolic diseases.





Similar content being viewed by others
References
Aleshin, S., and G. Reiser. 2013. Role of the peroxisome proliferator-activated receptors (PPAR)-α, β/δ and γ triad in regulation of reactive oxygen species signaling in brain. Biological Chemistry 394: 1553–1557.
Aleshin, S., M. Strokin, M. Sergeeva, and G. Reiser. 2013. Peroxisome proliferator-activated receptor (PPAR)β/δ, a possible nexus of PPARα- and PPARγ-dependent molecular pathways in neurodegenerative diseases: Review and novel hypotheses. Neurochemistry International 63: 322–330.
Barak, Y., M.C. Nelson, E.S. Ong, Y.Z. Jones, P.R. Lozano, K.R. Chien, A. Koder, and R.M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Molecular Cell 4: 585–595.
Barak, Y., D. Liao, W. He, E.S. Ong, M.C. Nelson, J.M. Olefsky, R. Boland, and R.M. Evans. 2002. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America 99: 303–308.
Bensinger, S.J., and P. Tontonoz. 2008. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454: 470–477.
Berger, J., and D.E. Moller. 2002. The mechanisms of action of PPARs. Annual Review of Medicine 53: 409–435.
Blunt, J.W., B.R. Copp, R.A. Keyzers, M.H. Munro, and M.R. Prinsep. 2013. Marine natural products. Natural Product Reports 30: 237–323.
Bonnard, I., S.B.J. Laulloo, N. Bontemps, B. Banaigs, and M. Aknin. 2010. New lobane and cembrane diterpenes from two comorian soft corals. Marine Drugs 8: 359–372.
Chung, J.H., A.Y. Seo, S.W. Chung, M.K. Kim, C. Leeuwenburgh, B.P. Yu, and H.Y. Chung. 2008. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Research Reviews 7: 126–136.
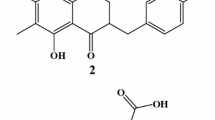
Cuong, N.X., N.P. Thao, B.T.T. Luyen, N.T.T. Ngan, D.T.T. Thuy, S.B. Song, N.H. Nam, P.V. Kiem, Y.H. Kim, and C.V. Minh. 2014. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chemical and Pharmaceutical Bulletin 62: 203–208.
Desvergne, B., L. Michalik, and W. Wahli. 2004. Befit or be sick: Peroxisome proliferatoractivated receptors are down the road. Molecular Endocrinology 18: 1321–1332.
Devchand, P.R., H. Keller, J.M. Peters, M. Vazquez, F.J. Gonzalez, and W. Wahli. 1996. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384: 39–43.
Evans, R.M. 1988. The steroid and thyroid hormone receptor superfamily. Science 240: 889–895.
Evans, R.M., G.D. Barish, and Y.X. Wang. 2004. PPARs and the complex journey to obesity. Nature Medicine 10: 355–361.
Gervois, P., J.C. Fruchartm, and B. Staels. 2005. Inflammation, dyslipidaemia, diabetes and PPARs: Pharmacological interest of dual PPARalpha and PPARgamma agonists. International Journal of Clinical Practice Supplement 143: 22–29.
Grimaldi, P.A. 2007. Regulatory functions of PPARβ in metabolism: Implications for the treatment of metabolic syndrome. Biochimica et Biophysica Acta 1771: 983–990.
Gross, B., and B. Staels. 2007. PPAR agonists: Multimodal drugs for the treatment of type-2 diabetes. Best Practice & Research Clinical Endocrinology & Metabolism 21: 687–710.
Grover, S., P. Kumar, K. Singh, V. Vikram, and R.D. Budhiraja. 2013. Possible beneficial effect of peroxisome proliferator-activated receptor (PPAR)-α and γ agonist against a rat model of oral dyskinesia. Pharmacology, Biochemistry and Behavior 111: 17–23.
Jawerbaum, A., and E. Capobianco. 2011. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta 25: S212–S217.
Kaplan, F., K.A. Majali, and D.J. Betteridge. 2001. PPARs, insulin resistance and type 2 diabetes. Journal of Cardiovascular Risk 405: 421–424.
Kersten, S., J. Seydoux, J.M. Peters, F.J. Gonzalez, B. Desvergne, and W. Wahli. 1999. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. The Journal of Clinical Investigation 103: 1489–1498.
Lee, C.H., P. Olson, A. Hevener, I. Mehl, L.W. Chong, J.M. Olefsky, F.J. Gonzalez, J. Ham, H. Kang, J.M. Peters, and R.M. Evans. 2006. PPARdelta regulates glucose metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America 103: 3444–3449.
Linford, N.J., R.P. Beyer, K. Gollahon, R.A. Krajcik, V.L. Malloy, V. Demas, G.C. Burmer, and P.S. Rabinovitch. 2007. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell 6: 673–688.
Moller, D.E. 2001. New drug targets for type II diabetes and the metabolic syndrome. Nature 414: 821–827.
Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M.R. Liu, D. Gorman, J. Wagner, S. Zurawski, Y.J. Liu, J.S. Abrams, K.W. Moore, D. Rennick, R.W. Malefyt, C. Hannum, J.F. Bazan, and R.A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725.
Park, M.H., J.Y. Park, H.J. Lee, D.H. Kim, K.W. Chung, D. Park, H.O. Jeong, H.R. Kim, C.H. Park, S.R. Kim, P. Chun, Y. Byun, H.R. Moon, and H.Y. Chung. 2013. The novel PPAR α/γ dual agonist MHY 966 modulates UVB-induced skin inflammation by inhibiting NF-κB activity. PLoS One 8: e76820.
Rashid, M.A., K.R. Gustafson, and M.R. Boyd. 2000. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. Journal of Natural Products 63: 531–533.
Rosen, E.D., and B.M. Spiegelman. 2000. Molecular regulation of adipogenesis. Annual Review of Cell and Developmental Biology 16: 145–171.
Shearer, B.G., and A.N. Billin. 2007. The next generation of PPAR drugs: Do we have the tools to find them? BBA Molecular and Cell Biology of Lipids 1771: 1082–1093.
Silva, F.M.C., J.C. Santo, J.L.O. Campos, A.C. Mafud, I. Polikarpov, A.C.M. Figueira, and A.S. Nascimento. 2013. Structure-based identification of novel PPAR gamma ligands. Bioorganic & Medicinal Chemistry Letters 23: 5795–5802.
Staels, B., W. Koenig, A. Habib, R. Merval, M. Lebret, I.P. Torra, P. Delerive, A. Fadel, G. Chinetti, J.C. Fruchart, J. Najib, J. Maclouf, and A. Tedgui. 1998. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 393: 790–793.
Straus, D.S., and C.K. Glass. 2007. Anti-inflammatory actions of PPAR ligands: New insights on cellular and molecular mechanisms. Trends in Immunology 28: 551–558.
Thao, N.P., N.H. Nam, N.X. Cuong, T.H. Quang, P.T. Tung, B.H. Tai, B.T.T. Luyen, D. Chae, S. Kim, Y.S. Koh, P.V. Kiem, C.V. Minh, and Y.H. Kim. 2012. Diterpenoids from the soft coral Sinularia maxima and their inhibitory effects on lipopolysaccharide-stimulated production of proinflammatory cytokines in bone marrow-derived dendritic cells. Chemical & Pharmaceutical Bulletin 60: 1581–1589.
Thao, N.P., N.H. Nam, N.X. Cuong, T.H. Quang, P.T. Tung, L.D. Dat, D. Chae, S. Kim, Y.S. Koh, P.V. Kiem, C.V. Minh, and Y.H. Kim. 2013a. Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorganic & Medicinal Chemistry Letters 23: 228–231.
Thao, N.P., N.H. Nam, N.X. Cuong, B.H. Tai, T.H. Quang, N.T.T. Ngan, B.T.T. Luyen, S.Y. Yang, C.H. Choi, S. Kim, D. Chae, Y.S. Koh, P.V. Kiem, C.V. Minh, and Y.H. Kim. 2013b. Steroidal constituents from the soft coral Sinularia dissecta and their inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Bulletin of the Korean Chemical Society 34: 949–952.
Thao, N.P., B.T.T. Luyen, N.T.T. Ngan, S.B. Song, N.X. Cuong, N.H. Nam, P.V. Kiem, Y.H. Kim, and C.V. Minh. 2014. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorganic & Medicinal Chemistry Letters 24: 228–232.
Villa, F.A., and L. Gerwick. 2010. Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacology and Immunotoxicology 32: 228–237.
Yang, L., J. Zhou, Q. Ma, C. Wang, K. Chen, W. Meng, Y. Yu, Z. Zhou, and X. Sun. 2013. Knockdown of PPAR δ gene promotes the growth of colon cancer and reduces the sensitivity to bevacizumab in nude mice model. PLoS One 8: e60715.
Acknowledgments
This study was supported by a grant from the Vietnam National Foundation for Science & Technology Development (Project No: 104.01-2012.37) and the framework of international cooperation program managed by National Research Foundation of Korea (2012-K2A1A2032970) and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2012-0006681), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Thao, N.P., Luyen, B.T.T., Ngan, N.T.T. et al. Peroxisome proliferator-activated receptor transactivational effects in HepG2 cells of cembranoids from the soft coral Lobophytum crassum Von Marenzeller . Arch. Pharm. Res. 38, 769–775 (2015). https://doi.org/10.1007/s12272-014-0382-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0382-9