Abstract
Calcium/calmodulin-dependent protein kinase II (CaMKII) has been demonstrated to be aberrantly activated in viral myocarditis (VMC), but the role of its subtype CaMKIIδ in VMC remains unclear.
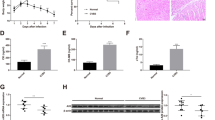
VMC mice and cardiomyocytes models were induced by Coxsackievirus B3 (CVB3) treatment. Mice that underwent sham surgery and saline-treated cardiomyocytes served as controls. Body weight, survival, left ventricular ejection fraction (LVEF), and fractional shortening (LVFS) were measured, and HE staining was performed to evaluate heart function in VMC mice model and sham control. Inflammation factors in serum or cell supernatant were detected by ELISA. Expressions of CaMKIIδ, Toll/interleukin-1 receptor domain containing adaptor protein (TIRAP), insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2), nuclear factor NF-kappaB (NF-κB) signals, and inflammation factors were examined by quantitative real time polymerase chain reaction (qRT-PCR) or western blot. CCK-8, EdU, and flow cytometry were used to evaluate cell behaviors. Co-immunoprecipitation (Co-IP), RNA immunoprecipitation (RIP), and RNA pull-down were utilized to validate molecule interaction. Methylated RNA immunoprecipitation (MeRIP) was performed to measure N6-methyladenosine (m6A) level of specific molecule.
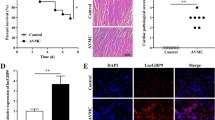
CaMKIIδ was upregulated in VMC mice and CVB3-treated primary cardiomyocytes, of which knockdown improved cell viability, proliferation, and suppressed cell apoptosis in vitro, thereby alleviating myocarditis in vivo. The stability of CaMKIIδ was attributed to the presence of IGF2BP2 through m6A modification. Loss of CaMKIIδ repressed NF-κB pathway via negatively and directly regulating TIRAP to be involved in inflammatory damage.
CaMKIIδ, stabilized by m6A reader IGF2BP2, modulated NF-κB pathway via interacting with TIRAP to alter cell viability, proliferation, and apoptosis, thereby affecting VMC outcome.
Graphical abstract







Similar content being viewed by others
Data Availability
All data collected and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- VMC:
-
viral myocarditis
- CaMKII:
-
calcium/calmodulin-dependent protein kinase II
- CVB3:
-
Coxsackievirus B3
- LVEF:
-
left ventricular ejection fraction
- LVFS:
-
left ventricular fractional shortening
- TIRAP:
-
Toll/interleukin-1 receptor domain containing adaptor protein (TIRAP)
- IGF2BP2:
-
insulin-like growth factor 2 mRNA binding protein 2
- NF-κB:
-
nuclear factor NF-kappaB
- qRT-PCR:
-
quantitative real-time polymerase chain reaction
- Co-IP:
-
co-immunoprecipitation
- RIP:
-
RNA immunoprecipitation
- MeRIP:
-
methylated RNA immunoprecipitation
- m6A:
-
N6-methyladenosine
- AMI:
-
acute myocardial infarction
- STAT3:
-
signal transducer and activator of transcription 3
- IL:
-
interleukin
- PBS:
-
phosphate-buffered saline
- METTL3:
-
methyltransferase 3
References
Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55.
Huber SA. Viral myocarditis and dilated cardiomyopathy: etiology and pathogenesis. Current Pharmac Design. 2016;22(4):408–26.
Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738–47.
Schultheiss HP, Baumeier C, Aleshcheva G, Bock CT, Escher F. Viral myocarditis-from pathophysiology to treatment. 2021;10 (22).
Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart. 2015;101(16):1332–44.
Blyszczuk P. Myocarditis in humans and in experimental animal models. Front Cardiovascular Med. 2019;6:64.
Si X, Luo H, Morgan A, Zhang J, Wong J, Yuan J, Esfandiarei M, Gao G, Cheung C, McManus BM. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. 2005;79 (22), 13875-13881.
Yuan JP, Zhao W, Wang HT, Wu KY, Li T, Guo XK, Tong SQ. Coxsackievirus B3-induced apoptosis and caspase-3. 2003;13 (3), 203-209.
Hu C, Zhou G, Liu K, Yin W, Zhou L, Wang J, Chen L, Zuo S, Xie Y, Zuo X. CaMKII as a key regulator of contrast-induced nephropathy through mPTP opening in HK-2 cells.2020;75 109734.
Lim JR, Lee HJ, Jung Y. H, Kim JS, Chae CW, Kim SY, Han HJ. Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation. 2020;18(1), 123.
Luo HM, Wu X, Liu WX, Wang LY, Sun HY, Zhu LY, Yang L. Calcitonin gene-related peptide attenuates angiotensin II-induced ROS-dependent apoptosis in vascular smooth muscle cells by inhibiting the CaMKII/CREB signalling pathway. Biochem Biophys Res Commun. 2020;521(2):285–9.
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J. et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. 2016;22(2), 175-182.
Gu J, Zhan Y, Zhuo L, Zhang Q, Li G, Li Q, Qi S, Zhu J, Lv Q, Shen Y. et al. Biological functions of m(6)A methyltransferases.2021;11 (1), 15.
Zhou T, Han D, Liu J, Shi J, Zhu P, Wang Y, Dong N. Factors influencing osteogenic differentiation of human aortic valve interstitial cells. J Thoracic Cardiovas Surg. 2021;161(2):e163–85.
Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM. et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. 2010;33 (5), 699-712.
Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–6.
Liu Y, Liu Z, Tang H, Shen Y, Gong Z, Xie N, Zhang X, Wang W, Kong W, Zhou Y. et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. 2019;317 (4), C762-C775.
Nie J, Ta N, Liu L, Shi G, Kang T, Zheng Z. Activation of CaMKII via ER-stress mediates coxsackievirus B3-induced cardiomyocyte apoptosis. 2020;44 (2), 488-498.
Rezkalla SH, Kloner RA. Viral myocarditis: 1917-2020: from the influenza A to the COVID-19 pandemics. 2021;31(3), 163-169.
Olejniczak M, Schwartz M, Webber E, Shaffer A, Perry TE. Viral myocarditis-incidence, diagnosis and management. 2020;34 (6), 1591-1601.
Sinagra G, Porcari A, Gentile P, Artico J, Fabris E, Bussani R, Merlo M. Viral presence-guided immunomodulation in lymphocytic myocarditis: an update. Eur J Heart Fail. 2021;23(2):211–6.
Cruz Junho CV, Trentin-Sonoda M, Alvim JM, Gaisler-Silva F, Carneiro-Ramos MS. Ca2+/Calmodulin-dependent kinase II delta B is essential for cardiomyocyte hypertrophy and complement gene expression after LPS and HSP60 stimulation in vitro. 2019;52(7), e8732.
Liu T, Zhang M, Niu H, Liu J, Ruilian M, Wang Y, Xiao Y, Xiao Z, Sun J, Dong Y. et al. Astragalus polysaccharide from Astragalus melittin ameliorates inflammation via suppressing the activation of TLR-4/NF-kappaB p65 signal pathway and protects mice from CVB3-induced virus myocarditis. 2019;126 179-186.
Belhaouane I, Hoffmann E, Chamaillard M, Brodin P, Machelart A. Paradoxical roles of the MAL/Tirap adaptor in pathologies. Front Immun. 2020;11:569127.
Evans S, Weinheimer CJ, Kovacs A, Williams JW, Randolph GJ, Jiang W, Barger PM, Mann DL. Ischemia reperfusion injury provokes adverse left ventricular remodeling in dysferlin-deficient hearts through a pathway that involves TIRAP dependent signaling. Scientific Rep. 2020;10(1):14129.
Berulava T, Buchholz E, Elerdashvili V, Pena T, Islam MR, Lbik D, Mohamed BA, Renner A, von Lewinski D, Sacherer M. et al. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. 2020;22 (1), 54-66.
Han Z, Wang X, Xu Z, Cao Y, Gong R, Yu Y, Yu Y, Guo X, Liu S, Yu M. et al. ALKBH5 regulates cardiomyocyte proliferation and heart regeneration by demethylating the mRNA of YTHDF1. 2021;11 (6), 3000-3016.
Li T, Zhuang Y, Yang W, Xie Y, Shang W, Su S, Dong X, Wu J, Jiang W, Zhou Y. et al. Silencing of METTL3 attenuates cardiac fibrosis induced by myocardial infarction via inhibiting the activation of cardiac fibroblasts. 2021;35 (2), e21162.
Nombela P, Miguel-Lopez B, Blanco S. The role of m(6)A, m(5)C and Psi RNA modifications in cancer: Novel therapeutic opportunities. 2021;20(1), 18.
Hosen MR, Militello G, Weirick T, Ponomareva Y, Dassanayaka S, Moore JB Th, Doring C, Wysoczynski M, Jones SP, Dimmeler S. et al. Airn Regulates Igf2bp2 Translation in Cardiomyocytes. 2018;122 (10), 1347-1353.
Qian B, Wang P, Zhang D, Wu L. m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2. 2021;7(1), 157.
Khyzha N, Khor M, DiStefano PV, Wang L, Matic L, Hedin U, Wilson MD, Maegdefessel L, Fish JE. Regulation of CCL2 expression in human vascular endothelial cells by a neighboring divergently transcribed long noncoding RNA. 2019;116 (33), 16410-16419.
Xiao K, Liu P, Yan P, Liu Y, Song L, Liu Y, Xie L. N6-methyladenosine reader YTH N6-methyladenosine RNA binding protein 3 or insulin like growth factor 2 mRNA binding protein 2 knockdown protects human bronchial epithelial cells from hypoxia/reoxygenation injury by inactivating p38 MAPK, AKT, ERK1/2, and NF-kappaB pathways. 2022;13 (5), 11973-11986.
Funding
The work was supported by Natural Science Foundation of Jiangxi Province (No. 20232BAB206015).
Author information
Authors and Affiliations
Contributions
Conception and design of study: JN; Acquisition of data: QX, WQ, RY; Analysis and interpretation of data: LL, TK; Drafting the manuscript: QX; Revising the manuscript critically for important intellectual content: JN.
Corresponding author
Ethics declarations
Ethics Approval
All the animal procedures were performed in accordance with the Guiding Principles in the Use and Care of Animals and approved by the Ethical Committee of The First Affiliated Hospital of Nanchang University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Joost Sluijter oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Knockdown of CaMKIIδ alleviates apoptosis and inflammation of cardiomyocytes in the cell model of VMC.
2. IGF2BP2 stabilizes CaMKIIδ by m6A modification.
3. CaMKIIδ interacts with and positively regulates TIRAP.
4. Knockdown of CaMKIIδ alleviates VMC through TIRAP inhibition in vivo.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Q., Liu, L., Qian, W. et al. CaMKIIδ, Stabilized by RNA N6-Methyladenosine Reader IGF2BP2, Boosts Coxsackievirus B3-Induced Myocardial Inflammation via Interacting with TIRAP. J. of Cardiovasc. Trans. Res. (2024). https://doi.org/10.1007/s12265-023-10478-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-023-10478-3