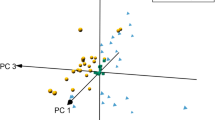
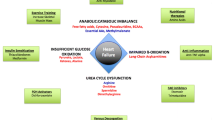
Abstract
Implantable cardioverter defibrillators (ICDs) reduce sudden cardiac death (SCD) when patients experience life-threatening ventricular arrhythmias (LTVA). However, current strategies determining ICD patient selection and risk stratification are inefficient. We used metabolomics to assess whether dysregulated metabolites are associated with LTVA and identify potential biomarkers. Baseline plasma samples were collected from 72 patients receiving ICDs. Over a median follow-up of 524.0 days (range 239.0–705.5), LTVA occurred in 23 (31.9%) patients (22 effective ICD treatments and 1 SCD). After confounding risk factors adjustment for age, smoking, secondary prevention, and creatine kinase MB, 23 metabolites were significantly associated with LTVA. Pathway analysis revealed LTVA associations with disrupted metabolism of glycine, serine, threonine, and branched chain amino acids. Pathway enrichment analysis identified a panel of 6 metabolites that potentially predicted LTVA, with an area under the receiver operating characteristic curve of 0.8. Future studies are necessary on biological mechanisms and potential clinical use.
Graphical abstract





Similar content being viewed by others
Abbreviations
- AF:
-
Atrial fibrillation
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- CABG:
-
Coronary artery bypass grafting
- ESI:
-
Electrospray ionization
- FAHFA:
-
Fatty acid esters of hydroxy fatty acids
- HR:
-
Hazard ratios
- ICD:
-
Implantable cardioverter defibrillator
- ICM:
-
Ischemic cardiomyopathy
- LASSO:
-
Least absolute shrinkage and selection operator
- LDL:
-
Low-density lipoprotein
- LPG:
-
Lysophosphatidylglycerol
- LTVA:
-
Life-threatening ventricular arrhythmia
- LVEDD:
-
Left ventricular end diastolic diameter
- LVEF:
-
Left ventricular ejection fraction
- LysoPG:
-
Lysophosphatidylglycerol
- MI:
-
Myocardial infarction
- NICM:
-
Non-ischemic cardiomyopathy
- NYHA:
-
New York Heart Association
- PA:
-
Phosphatidic acid
- PI:
-
Phosphatidylinositol
- ROC:
-
Receiver operating characteristic
- SCD:
-
Sudden cardiac death
- SM:
-
Sphingomyelin
- TC:
-
Total cholesterol
References
Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118(14):1497–518.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220. https://doi.org/10.1016/j.jacc.2017.10.054.
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62. https://doi.org/10.1016/j.jacc.2008.02.032.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. https://doi.org/10.1056/NEJMoa043399.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. https://doi.org/10.1056/NEJMoa013474.
Zwanziger J, Hall WJ, Dick AW, Zhao H, Mushlin AI, Hahn RM, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47(11):2310–8. https://doi.org/10.1016/j.jacc.2006.03.032.
Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150–7. https://doi.org/10.1016/j.jacc.2007.04.095.
Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51(3):288–96. https://doi.org/10.1016/j.jacc.2007.08.058.
Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, et al. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation. 2011;123(21):2423–30. https://doi.org/10.1161/CIRCULATIONAHA.110.959734.
Griffin JL, Atherton H, Shockcor J, Atzori L. Metabolomics as a tool for cardiac research. Nat Rev Cardiol. 2011;8(11):630–43. https://doi.org/10.1038/nrcardio.2011.138.
Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110–20. https://doi.org/10.1161/CIRCULATIONAHA.111.060368.
Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–9. https://doi.org/10.1038/nrm.2016.25.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):1677–749. https://doi.org/10.1016/j.jacc.2017.10.053.
Zhao J, Yang S, Jing R, Jin H, Hu Y, Wang J, et al. Plasma metabolomic profiles differentiate patients with dilated cardiomyopathy and ischemic cardiomyopathy. Front Cardiovasc Med. 2020;7:597546. https://doi.org/10.3389/fcvm.2020.597546.
Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinformatics. 2019;68(1):e86. https://doi.org/10.1002/cpbi.86.
Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221–30. https://doi.org/10.1056/NEJMoa1608029.
Barbier P, Mirea O, Cefalù C, Maltagliati A, Savioli G, Guglielmo M. Reliability and feasibility of longitudinal AFI global and segmental strain compared with 2D left ventricular volumes and ejection fraction: intra- and inter-operator, test-retest, and inter-cycle reproducibility. Eur Heart J Cardiovasc Imaging. 2015;16(6):642–52. https://doi.org/10.1093/ehjci/jeu274.
Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012;59(23):2075–9. https://doi.org/10.1016/j.jacc.2012.02.036.
Zhang S, Ching C-K, Huang D, Liu Y-B, Rodriguez-Guerrero DA, Hussin A, et al. Utilization of implantable cardioverter-defibrillators for the prevention of sudden cardiac death in emerging countries: improve SCA clinical trial. Heart Rhythm. 2020;17(3):468–75. https://doi.org/10.1016/j.hrthm.2019.09.023.
Martínez Y, Li X, Liu G, Bin P, Yan W, Más D, et al. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49(12):2091–8. https://doi.org/10.1007/s00726-017-2494-2.
Sanderson SM, Gao X, Dai Z, Locasale JW. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer. 2019;19(11):625–37. https://doi.org/10.1038/s41568-019-0187-8.
Lundberg P, Dudman NP, Kuchel PW, Wilcken DE. 1H NMR determination of urinary betaine in patients with premature vascular disease and mild homocysteinemia. Clin Chem. 1995;41(2):275–83.
Lever M, Atkinson W, Slow S, Chambers ST, George PM. Plasma and urine betaine and dimethylglycine variation in healthy young male subjects. Clin Biochem. 2009;42(7–8):706–12. https://doi.org/10.1016/j.clinbiochem.2009.02.001.
Barter PJ, Rye K-A. Homocysteine and cardiovascular disease: is HDL the link? Circ Res. 2006;99(6):565–6. https://doi.org/10.1161/01.RES.0000243583.39694.1f.
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9(1):e002314. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002314.
Svingen GFT, Ueland PM, Pedersen EKR, Schartum-Hansen H, Seifert R, Ebbing M, et al. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33(8):2041–8. https://doi.org/10.1161/ATVBAHA.113.301714.
Lever M, George PM, Elmslie JL, Atkinson W, Slow S, Molyneux SL, et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7(5):e37883. https://doi.org/10.1371/journal.pone.0037883.
Blüml S. In vivo quantitation of cerebral metabolite concentrations using natural abundance 13C MRS at 1.5 T. J Magnet Resonance (San Diego, Calif.: 1997). 1999;136(2):219–25. https://doi.org/10.1006/jmre.1998.1618.
Sahin I, Alkan A, Keskin L, Cikim A, Karakas HM, Firat AK, Sigirci A. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complicat. 2008;22(4):254–60. https://doi.org/10.1016/j.jdiacomp.2007.03.007.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81(2):89–131. https://doi.org/10.1016/j.pneurobio.2006.12.003.
Schuff N, Meyerhoff DJ, Mueller S, Chao L, Sacrey DT, Laxer K, Weiner MW. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–62; discussion 361-363. https://doi.org/10.1007/0-387-30172-0_17.
Daniele G, Campi B, Saba A, Codini S, Ciccarone A, Giusti L, et al. Plasma N-acetylaspartate is related to age, obesity, and glucose metabolism: effects of antidiabetic treatment and bariatric surgery. Front Endocrinol. 2020;11:216. https://doi.org/10.3389/fendo.2020.00216.
Lee CW, Lee JH, Lim TH, Yang HS, Hong MK, Song JK, et al. Prognostic significance of cerebral metabolic abnormalities in patients with congestive heart failure. Circulation. 2001;103(23):2784–7. https://doi.org/10.1161/01.cir.103.23.2784.
Lee CW, Lee JH, Kim JJ, Park SW, Hong MK, Kim ST, et al. Cerebral metabolic abnormalities in congestive heart failure detected by proton magnetic resonance spectroscopy. J Am Coll Cardiol. 1999;33(5):1196–202. https://doi.org/10.1016/s0735-1097(98)00701-3.
Makide K, Kitamura H, Sato Y, Okutani M, Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009;89(3–4):135–9. https://doi.org/10.1016/j.prostaglandins.2009.04.009.
Herrmann J, Mannheim D, Wohlert C, Versari D, Meyer FB, McConnell JP, et al. Expression of lipoprotein-associated phospholipase A(2) in carotid artery plaques predicts long-term cardiac outcome. Eur Heart J. 2009;30(23):2930–8. https://doi.org/10.1093/eurheartj/ehp309.
Park KS, Kim M-K, Im D-S, Bae Y-S. Effect of lysophosphatidylglycerol on several signaling molecules in OVCAR-3 human ovarian cancer cells: involvement of pertussis toxin-sensitive G-protein coupled receptor. Biochem Pharmacol. 2007;73(5):675–81. https://doi.org/10.1016/j.bcp.2006.11.010.
Walsh SK, Hector EE, Andréasson A-C, Jönsson-Rylander A-C, Wainwright CL. GPR55 deletion in mice leads to age-related ventricular dysfunction and impaired adrenoceptor-mediated inotropic responses. PLoS One. 2014;9(9):e108999. https://doi.org/10.1371/journal.pone.0108999.
Zegarlińska J, Piaścik M, Sikorski AF, Czogalla A. Phosphatidic acid - a simple phospholipid with multiple faces. Acta Biochim Pol. 2018;65(2):163–71. https://doi.org/10.18388/abp.2018_2592.
Takahashi H, Takeishi Y, Seidler T, Arimoto T, Akiyama H, Hozumi Y, et al. Adenovirus-mediated overexpression of diacylglycerol kinase-zeta inhibits endothelin-1-induced cardiomyocyte hypertrophy. Circulation. 2005;111(12):1510–6. https://doi.org/10.1161/01.CIR.0000159339.00703.22.
Arimoto T, Takeishi Y, Takahashi H, Shishido T, Niizeki T, Koyama Y, et al. Cardiac-specific overexpression of diacylglycerol kinase zeta prevents Gq protein-coupled receptor agonist-induced cardiac hypertrophy in transgenic mice. Circulation. 2006;113(1):60–6. https://doi.org/10.1161/CIRCULATIONAHA.105.560771.
Dhalla NS, Xu YJ, Sheu SS, Tappia PS, Panagia V. Phosphatidic acid: a potential signal transducer for cardiac hypertrophy. J Mol Cell Cardiol. 1997;29(11):2865–71. https://doi.org/10.1006/jmcc.1997.0522.
Waugh M. Measuring phosphatidylinositol generation on biological membranes. Methods Mol Biol. 2016;1376:239–46. https://doi.org/10.1007/978-1-4939-3170-5_20.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. https://doi.org/10.1038/nrg1879.
Ghigo A, Laffargue M, Li M, Hirsch E. PI3K and calcium signaling in cardiovascular disease. Circ Res. 2017;121(3):282–92. https://doi.org/10.1161/CIRCRESAHA.117.310183.
Acknowledgements
We appreciate the work of our clinical colleagues who performed clinical data collection and all individuals who participated in this study.
Funding
This research was funded by Shengwen Yang of the Beijing Chaoyang Hospital Golden Seeds Foundation (CYJZ202103), Wei Hua of the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2017-I2M-1-009), and the National Natural Science Foundation of China (81570370).
Author information
Authors and Affiliations
Contributions
Conceptualization, Shengwen Yang and Junhan Zhao; methodology, Hongxia Niu; software, Xi Liu; validation, Chi Cai and Jing Wang; formal analysis, Shengwen Yang; investigation, Shengwen Yang; resources, Xi Liu and Min Gu; data curation, Junhan Zhao; writing, original draft preparation, Junhan Zhao; writing, review and editing, Liang Chen; visualization, Junhan Zhao; supervision, Wei Hua and Liang Chen; project administration, Junhan Zhao; funding acquisition, Shengwen Yang and Wei Hua. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Fuwai. Informed consent was obtained from all participants in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shengwen Yang, Junhan Zhao, and Xi Liu are lead co-authors in the making of this manuscript.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, S., Zhao, J., Liu, X. et al. Metabolomics Profiling Predicts Ventricular Arrhythmia in Patients with an Implantable Cardioverter Defibrillator. J. of Cardiovasc. Trans. Res. 17, 91–101 (2024). https://doi.org/10.1007/s12265-023-10413-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10413-6