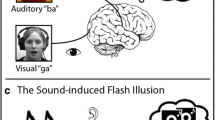
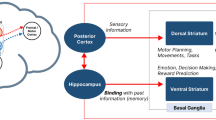
Abstract
Audiovisual integration is a vital information process involved in cognition and is closely correlated with aging and Alzheimer’s disease (AD). In this review, we evaluated the altered audiovisual integrative behavioral symptoms in AD. We further analyzed the relationships between AD pathologies and audiovisual integration alterations bidirectionally and suggested the possible mechanisms of audiovisual integration alterations underlying AD, including the imbalance between energy demand and supply, activity-dependent degeneration, disrupted brain networks, and cognitive resource overloading. Then, based on the clinical characteristics including electrophysiological and imaging data related to audiovisual integration, we emphasized the value of audiovisual integration alterations as potential biomarkers for the early diagnosis and progression of AD. We also highlighted that treatments targeted audiovisual integration contributed to widespread pathological improvements in AD animal models and cognitive improvements in AD patients. Moreover, investigation into audiovisual integration alterations in AD also provided new insights and comprehension about sensory information processes.

Similar content being viewed by others
References
Haykin S, Chen Z. The cocktail party problem. Neural Comput 2005, 17: 1875–1902.
McGurk H, MacDonald J. Hearing lips and seeing voices. Nature 1976, 264: 746–748.
Shams L, Kamitani Y, Shimojo S. Visual illusion induced by sound. Brain Res Cogn Brain Res 2002, 14: 147–152.
Bizley JK, Maddox RK, Lee AKC. Defining auditory-visual objects: Behavioral tests and physiological mechanisms. Trends Neurosci 2016, 39: 74–85.
Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: A behavioral and electrophysiological study. J Cogn Neurosci 1999, 11: 473–490.
Jensen A, Merz S, Spence C, Frings C. Perception it is: Processing level in multisensory selection. Atten Percept Psychophys 2020, 82: 1391–1406.
Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: Patchy organization within human STS multisensory cortex. Nat Neurosci 2004, 7: 1190–1192.
Musacchia G, Sams M, Nicol T, Kraus N. Seeing speech affects acoustic information processing in the human brainstem. Exp Brain Res 2006, 168: 1–10.
Nath AR, Beauchamp MS. Dynamic changes in superior temporal sulcus connectivity during perception of noisy audiovisual speech. J Neurosci 2011, 31: 1704–1714.
Yovel G, O’Toole AJ. Recognizing people in motion. Trends Cogn Sci 2016, 20: 383–395.
Calvert GA, Campbell R, Brammer MJ. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol 2000, 10: 649–657.
Noesselt T, Rieger JW, Schoenfeld MA, Kanowski M, Hinrichs H, Heinze HJ. Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. J Neurosci 2007, 27: 11431–11441.
Bernstein LE, Auer ET Jr, Wagner M, Ponton CW. Spatiotemporal dynamics of audiovisual speech processing. NeuroImage 2008, 39: 423–435.
Bushara KO, Grafman J, Hallett M. Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci 2001, 21: 300–304.
Shan L, Yuan L, Zhang B, Ma J, Xu X, Gu F, et al. Neural Integration of Audiovisual Sensory Inputs in Macaque Amygdala and Adjacent Regions. Neurosci Bull 2023, https://doi.org/10.1007/s12264-023-01043-8.
Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron 2008, 57: 11–23.
Bernstein LE, Liebenthal E. Neural pathways for visual speech perception. Front Neurosci 2014, 8: 386.
Macaluso E, George N, Dolan R, Spence C, Driver J. Spatial and temporal factors during processing of audiovisual speech: A PET study. Neuroimage 2004, 21: 725–732.
Bizley JK, Nodal FR, Bajo VM, Nelken I, King AJ. Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb Cortex 2007, 17: 2172–2189.
Pekkola J, Ojanen V, Autti T, Jääskeläinen IP, Möttönen R, Tarkiainen A, et al. Primary auditory cortex activation by visual speech: An fMRI study at 3 T. Neuroreport 2005, 16: 125–128.
Hertrich I, Mathiak K, Lutzenberger W, Ackermann H. Time course of early audiovisual interactions during speech and nonspeech central auditory processing: A magnetoencephalography study. J Cogn Neurosci 2009, 21: 259–274.
Ding H, Qin W, Liang M, Ming D, Wan B, Li Q, et al. Cross-modal activation of auditory regions during visuo-spatial working memory in early deafness. Brain 2015, 138: 2750–2765.
Baier B, Kleinschmidt A, Müller NG. Cross-modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. J Neurosci 2006, 26: 12260–12265.
Dhamala M, Assisi CG, Jirsa VK, Steinberg FL, Kelso JA. Multisensory integration for timing engages different brain networks. Neuroimage 2007, 34: 764–773.
Senkowski D, Schneider TR, Foxe JJ, Engel AK. Crossmodal binding through neural coherence: Implications for multisensory processing. Trends Neurosci 2008, 31: 401–409.
Yan T, Bi X, Zhang M, Wang W, Yao Z, Yang W, et al. Age-related oscillatory theta modulation of multisensory integration in frontocentral regions. Neuroreport 2016, 27: 796–801.
Keller AS, Payne L, Sekuler R. Characterizing the roles of alpha and theta oscillations in multisensory attention. Neuropsychologia 2017, 99: 48–63.
VanRullen R. Perceptual cycles. Trends Cogn Sci 2016, 20: 723–735.
Bastiaansen M, Berberyan H, Stekelenburg JJ, Schoffelen JM, Vroomen J. Are alpha oscillations instrumental in multisensory synchrony perception? Brain Res 2020, 1734: 146744.
Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 2000, 97: 1867–1872.
Keil J, Senkowski D. Neural oscillations orchestrate multisensory processing. Neuroscientist 2018, 24: 609–626.
Senkowski D, Talsma D, Herrmann CS, Woldorff MG. Multisensory processing and oscillatory gamma responses: Effects of spatial selective attention. Exp Brain Res 2005, 166: 411–426.
Mercier MR, Foxe JJ, Fiebelkorn IC, Butler JS, Schwartz TH, Molholm S. Auditory-driven phase reset in visual cortex: Human electrocorticography reveals mechanisms of early multisensory integration. Neuroimage 2013, 79: 19–29.
Bauer AR, Debener S, Nobre AC. Synchronisation of neural oscillations and cross-modal influences. Trends Cogn Sci 2020, 24: 481–495.
Cooke J, Poch C, Gillmeister H, Costantini M, Romei V. Oscillatory properties of functional connections between sensory areas mediate cross-modal illusory perception. J Neurosci 2019, 39: 5711–5718.
Mercier MR, Molholm S, Fiebelkorn IC, Butler JS, Schwartz TH, Foxe JJ. Neuro-oscillatory phase alignment drives speeded multisensory response times: An electro-corticographic investigation. J Neurosci 2015, 35: 8546–8557.
Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 2010, 14: 506–515.
Rosemann S, Wefel IM, Elis V, Fahle M. Audio-visual interaction in visual motion detection: Synchrony versus Asynchrony. J Optom 2017, 10: 242–251.
Watanabe H, Bagarinao E, Maesawa S, Hara K, Kawabata K, Ogura A, et al. Characteristics of neural network changes in normal aging and early dementia. Front Aging Neurosci 2021, 13: 747359.
Albouy P, Martinez-Moreno ZE, Hoyer RS, Zatorre RJ, Baillet S. Supramodality of neural entrainment: Rhythmic visual stimulation causally enhances auditory working memory performance. Sci Adv 2022, 8: eabj9782.
Zhang L, Du Y. Lip movements enhance speech representations and effective connectivity in auditory dorsal stream. Neuroimage 2022, 257: 119311.
Holmes NP. The law of inverse effectiveness in neurons and behaviour: Multisensory integration versus normal variability. Neuropsychologia 2007, 45: 3340–3345.
Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci 2004, 8: 162–169.
Cienkowski KM, Carney AE. Auditory-visual speech perception and aging. Ear Hear 2002, 23: 439–449.
Alsius A, Navarra J, Campbell R, Soto-Faraco S. Audiovisual integration of speech falters under high attention demands. Curr Biol 2005, 15: 839–843.
Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiol Aging 2006, 27: 1155–1163.
Mozolic JL, Hugenschmidt CE, Peiffer AM, Laurienti PJ. Modality-specific selective attention attenuates multisensory integration. Exp Brain Res 2008, 184: 39–52.
de Dieuleveult AL, Siemonsma PC, van Erp JB, Brouwer AM. Effects of aging in multisensory integration: A systematic review. Front Aging Neurosci 2017, 9: 80.
Ren Y, Li S, Zhao N, Hou Y, Wang T, Ren Y, et al. Auditory attentional load attenuates age-related audiovisual integration: An EEG study. Neuropsychologia 2022, 174: 108346.
Mozolic JL, Hugenschmidt CE, Peiffer AM, Laurienti PJ. Multisensory integration and aging. CRC Press/Taylor & Francis 2012
Peiffer AM, Mozolic JL, Hugenschmidt CE, Laurienti PJ. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport 2007, 18: 1077–1081.
Diederich A, Colonius H, Schomburg A. Assessing age-related multisensory enhancement with the time-window-of-integration model. Neuropsychologia 2008, 46: 2556–2562.
Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: Evidence for deficits in inhibitory control and sensory memory. Psychol Aging 1999, 14: 507–519.
Talsma D, Doty TJ, Woldorff MG. Selective attention and audiovisual integration: Is attending to both modalities a prerequisite for early integration? Cereb Cortex 2007, 17: 679–690.
Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia 2006, 44: 2564–2568.
Guerreiro MJ, Murphy DR, Van Gerven PW. The role of sensory modality in age-related distraction: A critical review and a renewed view. Psychol Bull 2010, 136: 975–1022.
Festa EK, Katz AP, Ott BR, Tremont G, Heindel WC. Dissociable effects of aging and mild cognitive impairment on bottom-up audiovisual integration. J Alzheimers Dis 2017, 59: 155–167.
Hugenschmidt CE, Mozolic JL, Tan H, Kraft RA, Laurienti PJ. Age-related increase in cross-sensory noise in resting and steady-state cerebral perfusion. Brain Topogr 2009, 21: 241–251.
Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci 2006, 18: 227–241.
Stevens WD, Hasher L, Chiew KS, Grady CL. A neural mechanism underlying memory failure in older adults. J Neurosci 2008, 28: 12820–12824.
Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci 2006, 9: 971–978.
Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: Change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 2003, 100: 14504–14509.
Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. NeuroImage 2007, 38: 640–648.
Basharat A, Thayanithy A, Barnett-Cowan M. A scoping review of audiovisual integration methodology: Screening for auditory and visual impairment in younger and older adults. Front Aging Neurosci 2021, 13: 772112.
Wu J, Yang J, Yu Y, Li Q, Nakamura N, Shen Y, et al. Delayed audiovisual integration of patients with mild cognitive impairment and Alzheimer’s disease compared with normal aged controls. J Alzheimers Dis 2012, 32: 317–328.
Chauvin A, Baum S, Phillips NA. Individuals with mild cognitive impairment and alzheimer’s disease benefit from audiovisual speech cues and supportive sentence context. J Speech Lang Hear Res 2021, 64: 1550–1559.
Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: Relationship to episodic and semantic memory impairment. Neuropsychologia 2000, 38: 252–271.
Chan JS, Kaiser J, Brandl M, Matura S, Prvulovic D, Hogan MJ, et al. Expanded temporal binding windows in people with mild cognitive impairment. Curr Alzheimer Res 2015, 12: 61–68.
Byeon G, Oh GH, Jhoo JH, Jang JW, Bae JB, Han JW, et al. Dual sensory impairment and cognitive impairment in the Korean longitudinal elderly cohort. Neurology 2021, 96: e2284–e2295.
Lin MY, Gutierrez PR, Stone KL, Yaffe K, Ensrud KE, Fink HA, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004, 52: 1996–2002.
Brenowitz WD, Kaup AR, Yaffe K. Incident dementia and faster rates of cognitive decline are associated with worse multisensory function summary scores. Alzheimers Dement 2020, 16: 1384–1392.
Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci 2019, 74: 890–896.
Pinto JM, Wroblewski KE, Huisingh-Scheetz M, Correia C, Lopez KJ, Chen RC, et al. Global sensory impairment predicts morbidity and mortality in older U.S. adults. J Am Geriatr Soc 2017, 65: 2587–2595.
Hughes TF, Ganguli M. Modifiable midlife risk factors for late-life cognitive impairment and dementia. Curr Psychiatry Rev 2009, 5: 73–92.
Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology 2004, 63: 2322–2326.
Long JM, Holtzman DM. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179: 312–339.
Jia J, Xu J, Liu J, Wang Y, Wang Y, Cao Y, et al. Comprehensive management of daily living activities, behavioral and psychological symptoms, and cognitive function in patients with Alzheimer’s disease: A Chinese consensus on the comprehensive management of Alzheimer’s disease. Neurosci Bull 2021, 37: 1025–1038.
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1991, 1: 103–116.
Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: A quantitative study of visual and auditory cortices. J Neurosci 1987, 7: 1799–1808.
Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging 1997, 12: 12–21.
Delbeuck X, Collette F, Van der Linden M. Is Alzheimer’s disease a disconnection syndrome? Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia 2007, 45: 3315–3323.
Schultz SA, Gordon BA, Mishra S, Su Y, Perrin RJ, Cairns NJ, et al. Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol Aging 2018, 72: 177–185.
Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 1997, 41: 17–24.
Buldyrev SV, Cruz L, Gomez-Isla T, Gomez-Tortosa E, Havlin S, Le R, et al. Description of microcolumnar ensembles in association cortex and their disruption in Alzheimer and Lewy body dementias. Proc Natl Acad Sci U S A 2000, 97: 5039–5043.
Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 1999, 52: 1158–1165.
Griffiths TD, Lad M, Kumar S, Holmes E, McMurray B, Maguire EA, et al. How can hearing loss cause dementia? Neuron 2020, 108: 401–412.
Camandola S, Mattson MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J 2017, 36: 1474–1492.
Mattson MP, Arumugam TV. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab 2018, 27: 1176–1199.
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005, 102: 5618–5623.
Pan X, Sang S, Zhong C. Brain energy improvement as an emerging approach for alzheimer’s disease treatment. Neurosci Bull 2021, 37: 892–893.
Wang R, Reddy PH. Role of glutamate and NMDA receptors in alzheimer’s disease. J Alzheimers Dis 2017, 57: 1041–1048.
Liu YZ, Wang Y, Tang W, Zhu JY, Wang Z. NMDA receptor-gated visual responses in hippocampal CA1 neurons. J Physiol 2018, 596: 1965–1979.
Truszkowski TL, Carrillo OA, Bleier J, Ramirez-Vizcarrondo CM, Felch DL, McQuillan M, et al. A cellular mechanism for inverse effectiveness in multisensory integration. Elife 2017, 6: e25392.
Beckmann D, Feldmann M, Shchyglo O, Manahan-Vaughan D. Hippocampal synaptic plasticity, spatial memory, and neurotransmitter receptor expression are profoundly altered by gradual loss of hearing ability. Cereb Cortex 2020, 30: 4581–4596.
de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 2012, 8: e1002582.
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 2011, 14: 750–756.
Jack CR, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, et al. The bivariate distribution of amyloid-β and tau: Relationship with established neurocognitive clinical syndromes. Brain 2019, 142: 3230–3242.
Leal SL, Landau SM, Bell RK, Jagust WJ. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife 2017, 6: e22978.
Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 2016, 19: 1085–1092.
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142: 387–397.
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62: 42–52.
Powers AR III, Hevey MA, Wallace MT. Neural correlates of multisensory perceptual learning. J Neurosci 2012, 32: 6263–6274.
Engelhardt E, Moreira DM, Laks J. The brain subcortical white matter and aging: A quantitative fractional anisotropy analysis. Dement Neuropsychol 2009, 3: 228–233.
Esposito F, Aragri A, Latorre V, Popolizio T, Scarabino T, Cirillo S, et al. Does the default-mode functional connectivity of the brain correlate with working-memory performances? Arch Ital Biol 2009, 147: 11–20.
Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 2007, 104: 18760–18765.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 2004, 101: 4637–4642.
Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, et al. Altered default mode network connectivity in Alzheimer’s disease—a resting functional MRI and Bayesian network study. Hum Brain Mapp 2011, 32: 1868–1881.
Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, et al. Resting brain connectivity: Changes during the progress of Alzheimer disease. Radiology 2010, 256: 598–606.
Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol 2004, 1: 351–363.
Yang H, Luo Y, Hu Q, Tian X, Wen H. Benefits in Alzheimer’s disease of sensory and multisensory stimulation. J Alzheimers Dis 2021, 82: 463–484.
Kelly C, Castellanos FX. Strengthening connections: Functional connectivity and brain plasticity. Neuropsychol Rev 2014, 24: 63–76.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012, 11: 1006–1012.
Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog Neurobiol 2009, 89: 369–382.
Sale A, Berardi N, Maffei L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol Rev 2014, 94: 189–234.
Kozubski W, Ong K, Waleszczyk W, Zabel M, Dorszewska J. Molecular factors mediating neural cell plasticity changes in dementia brain diseases. Neural Plast 2021, 2021: 8834645.
Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci 2013, 17: 502–509.
Archibald LM, Levee T, Olino T. Attention allocation: Relationships to general working memory or specific language processing. J Exp Child Psychol 2015, 139: 83–98.
Gan L, Wu J, Dai J, Funahashi S. The mechanism for allocating limited working memory resources in multitasking. Neurosci Bull 2022, 38: 829–833.
de Haan W, van Straaten ECW, Gouw AA, Stam CJ. Altering neuronal excitability to preserve network connectivity in a computational model of Alzheimer’s disease. PLoS Comput Biol 2017, 13: e1005707.
Li S, Sheng ZH. Energy matters: Presynaptic metabolism and the maintenance of synaptic transmission. Nat Rev Neurosci 2022, 23: 4–22.
Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement (Amst) 2018, 10: 196–209.
Cornelio P, Velasco C, Obrist M. Multisensory integration as per technological advances: A review. Front Neurosci 2021, 15: 652611.
Golob EJ, Miranda GG, Johnson JK, Starr A. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging 2001, 22: 755–763.
Ranasinghe KG, Petersen C, Kudo K, Mizuiri D, Rankin KP, Rabinovici GD, et al. Reduced synchrony in alpha oscillations during life predicts post mortem neurofibrillary tangle density in early-onset and atypical Alzheimer’s disease. Alzheimers Dement 2021, 17: 2009–2019.
Ranasinghe KG, Cha J, Iaccarino L, Hinkley LB, Beagle AJ, Pham J, et al. Neurophysiological signatures in Alzheimer’s disease are distinctly associated with TAU, amyloid-β accumulation, and cognitive decline. Sci Transl Med 2020, 12: eaaz4069.
Jafari Z, Kolb BE, Mohajerani MH. Neural oscillations and brain stimulation in Alzheimer’s disease. Prog Neurobiol 2020, 194: 101878.
Kocagoncu E, Quinn A, Firouzian A, Cooper E, Greve A, Gunn R, et al. Tau pathology in early Alzheimer’s disease is linked to selective disruptions in neurophysiological network dynamics. Neurobiol Aging 2020, 92: 141–152.
Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol 2018, 52: 182–187.
Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci 2016, 17: 239–249.
Tanaka H, Adachi H, Ukita N, Ikeda M, Kazui H, Kudo T, et al. Detecting dementia through interactive computer avatars. IEEE J Transl Eng Health Med 2017, 5: 2200111.
Stickel S, Weismann P, Kellermann T, Regenbogen C, Habel U, Freiherr J, et al. Audio-visual and olfactory-visual integration in healthy participants and subjects with autism spectrum disorder. Hum Brain Mapp 2019, 40: 4470–4486.
Liu T, Sachdev PS, Lipnicki DM, Jiang J, Cui Y, Kochan NA, et al. Longitudinal changes in sulcal morphology associated with late-life aging and MCI. Neuroimage 2013, 74: 337–342.
Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol 2000, 47: 430–439.
Pietrini P, Dani A, Furey ML, Alexander GE, Freo U, Grady CL, et al. Low glucose metabolism during brain stimulation in older Down’s syndrome subjects at risk for Alzheimer’s disease prior to dementia. Am J Psychiatry 1997, 154: 1063–1069.
Bokde AL, Teipel SJ, Drzezga A, Thissen J, Bartenstein P, Dong W, et al. Association between cognitive performance and cortical glucose metabolism in patients with mild Alzheimer’s disease. Dement Geriatr Cogn Disord 2005, 20: 352–357.
Pietrini P, Alexander GE, Furey ML, Dani A, Mentis MJ, Horwitz B, et al. Cerebral metabolic response to passive audiovisual stimulation in patients with Alzheimer’s disease and healthy volunteers assessed by PET. J Nucl Med 2000, 41: 575–583.
Drzezga A, Grimmer T, Peller M, Wermke M, Siebner H, Rauschecker JP, et al. Impaired cross-modal inhibition in Alzheimer disease. PLoS Med 2005, 2: e288.
Rees A. Aligning maps of visual and auditory space. Sensory maps. Curr Biol 1996, 6: 955–958.
Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature 1999, 402: 529–533.
Goto S, Kamal N, Puzio H, Kobylarz F, Herrup K. Differential responses of individuals with late-stage dementia to two novel environments: A multimedia room and an interior garden. J Alzheimers Dis 2014, 42: 985–998.
Gueib C, Pop A, Bannay A, Nassau E, Fescharek R, Gil R, et al. Impact of a healing garden on self-consciousness in patients with advanced Alzheimer’s disease: An exploratory Study1. J Alzheimers Dis 2020, 75: 1283–1300.
Rivasseau Jonveaux T, Batt M, Fescharek R, Benetos A, Trognon A, Bah Chuzeville S, et al. Healing gardens and cognitive behavioral units in the management of Alzheimer’s disease patients: The Nancy experience. J Alzheimers Dis 2013, 34: 325–338.
Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: A systematic review. Am J Geriatr Psychiatry 2012, 20: 549–564.
Lee LP, Har AW, Ngai CH, Lai DWL, Lam BY, Chan CC. Audiovisual integrative training for augmenting cognitive- motor functions in older adults with mild cognitive impairment. BMC Geriatr 2020, 20: 64.
Optale G, Urgesi C, Busato V, Marin S, Piron L, Priftis K, et al. Controlling memory impairment in elderly adults using virtual reality memory training: A randomized controlled pilot study. Neurorehabil Neural Repair 2010, 24: 348–357.
Cimenser A, Hempel E, Travers T, Strozewski N, Martin K, Malchano Z, et al. Sensory-evoked 40-hz gamma oscillation improves sleep and daily living activities in alzheimer’s disease patients. Front Syst Neurosci 2021, 15: 746859.
Chung JC, Lai CK, Chung PM, French HP. Snoezelen for dementia. Cochrane Database Syst Rev 2002: CD003152.
Zhang L, Wang X, Alain C, Du Y. Successful aging of musicians: Preservation of sensorimotor regions aids audiovisual speech-in-noise perception. Sci Adv 2023, 9: eadg7056.
Matziorinis AM, Koelsch S. The promise of music therapy for Alzheimer’s disease: A review. Ann N Y Acad Sci 2022, 1516: 11–17.
Zheng J, Li Y. Compensation for neurodegeneration by hippocampal neurogenesis in Alzheimer’s disease: Where is the way? Neurosci Bull 2021, 37: 885–888.
Benoit M, Guerchouche R, Petit PD, Chapoulie E, Manera V, Chaurasia G, et al. Is it possible to use highly realistic virtual reality in the elderly? A feasibility study with image-based rendering. Neuropsychiatr Dis Treat 2015, 11: 557–563.
Manera V, Chapoulie E, Bourgeois J, Guerchouche R, David R, Ondrej J, et al. A feasibility study with image-based rendered virtual reality in patients with mild cognitive impairment and dementia. PLoS One 2016, 11: e0151487.
Yan M, Zhao Y, Meng Q, Wang S, Ding Y, Liu Q, et al. Effects of virtual reality combined cognitive and physical interventions on cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis. Ageing Res Rev 2022, 81: 101708.
Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am J Psychiatry 2017, 174: 329–340.
Doniger GM, Beeri MS, Bahar-Fuchs A, Gottlieb A, Tkachov A, Kenan H, et al. Virtual reality-based cognitive-motor training for middle-aged adults at high Alzheimer’s disease risk: A randomized controlled trial. Alzheimers Dement (N Y) 2018, 4: 118–129.
Pantoni L, Poggesi A, Diciotti S, Valenti R, Orsolini S, Della Rocca E, et al. Effect of attention training in mild cognitive impairment patients with subcortical vascular changes: The RehAtt study. J Alzheimers Dis 2017, 60: 615–624.
Posporelis S, David AS, Ashkan K, Shotbolt P. Deep brain stimulation of the memory circuit: Improving cognition in Alzheimer’s disease. J Alzheimers Dis 2018, 64: 337–347.
Lam J, Lee J, Liu CY, Lozano AM, Lee DJ. Deep brain stimulation for Alzheimer’s disease: Tackling circuit dysfunction. Neuromodulation 2021, 24: 171–186.
Lin Y, Jiang WJ, Shan PY, Lu M, Wang T, Li RH, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J Neurol Sci 2019, 398: 184–191.
Rajji TK. Transcranial magnetic and electrical stimulation in Alzheimer’s disease and mild cognitive impairment: A review of randomized controlled trials. Clin Pharmacol Ther 2019, 106: 776–780.
Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 2019, 177: 256-271.e22.
Smith BC, D’Amico M. Sensory-based interventions for adults with dementia and Alzheimer’s disease: A scoping review. Occup Ther Health Care 2020, 34: 171–201.
Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DNW, et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 2019, 102: 929-943.e8.
Chan D, Suk HJ, Jackson B, Milman NP, Stark D, Beach SD, et al. Induction of specific brain oscillations may restore neural circuits and be used for the treatment of Alzheimer’s disease. J Intern Med 2021, 290: 993–1009.
He Q, Colon-Motas KM, Pybus AF, Piendel L, Seppa JK, Walker ML, et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement (N Y) 2021, 7: e12178.
Clouter A, Shapiro KL, Hanslmayr S. Theta phase synchronization is the glue that binds human associative memory. Curr Biol 2017, 27: 3143-3148.e6.
Acknowledgements
This review was supported by the National Key Research and Development Program of China (2022YFC3602600), National Natural Science Foundation of China (82220108009, 81970996), and STI2030-Major Projects (2021ZD0201801).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, Z., Wei, T. et al. Alterations of Audiovisual Integration in Alzheimer’s Disease. Neurosci. Bull. 39, 1859–1872 (2023). https://doi.org/10.1007/s12264-023-01125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-023-01125-7