Abstract
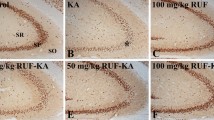
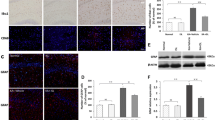
An increased level of reactive oxygen species is a key factor in neuronal apoptosis and epileptic seizures. Irisin reportedly attenuates the apoptosis and injury induced by oxidative stress. Therefore, we evaluated the effects of exogenous irisin in a kainic acid (KA)-induced chronic spontaneous epilepsy rat model. The results indicated that exogenous irisin significantly attenuated the KA-induced neuronal injury, learning and memory defects, and seizures. Irisin treatment also increased the levels of brain-derived neurotrophic factor (BDNF) and uncoupling protein 2 (UCP2), which were initially reduced following KA administration. Furthermore, the specific inhibitor of UCP2 (genipin) was administered to evaluate the possible protective mechanism of irisin. The reduced apoptosis, neurodegeneration, and spontaneous seizures in rats treated with irisin were significantly reversed by genipin administration. Our findings indicated that neuronal injury in KA-induced chronic epilepsy might be related to reduced levels of BDNF and UCP2. Moreover, our results confirmed the inhibition of neuronal injury and epileptic seizures by exogenous irisin. The protective effects of irisin may be mediated through the BDNF-mediated UCP2 level. Our results thus highlight irisin as a valuable therapeutic strategy against neuronal injury and epileptic seizures.







Similar content being viewed by others
References
Wurina S, Zang YF, Zhao SG. Resting-state fMRI studies in epilepsy. Neurosci Bull 2012, 28: 449–455.
Liu YQ, Yu F, Liu WH, He XH, Peng BW. Dysfunction of hippocampal interneurons in epilepsy. Neurosci Bull 2014, 30: 985–998.
Mishra CB, Kumari S, Prakash A, Yadav R, Tiwari AK, Pandey P. Discovery of novel Methylsulfonyl phenyl derivatives as potent human Cyclooxygenase-2 inhibitors with effective anticonvulsant action: Design, synthesis, in-silico, in-vitro and in-vivo evaluation. Eur J Med Chem 2018, 151: 520–532.
Ngugi AK, Bottomley C, Fegan G, Chengo E, Odhiambo R, Bauni E, et al. Premature mortality in active convulsive epilepsy in rural Kenya: Causes and associated factors. Neurology 2014, 82: 582–589.
Eid T, Lee TSW, Patrylo P, Zaveri HP. Astrocytes and glutamine synthetase in epileptogenesis. J Neurosci Res 2019, 97: 1345–1362.
Jin MM, Chen Z. Role of gap junctions in epilepsy. Neurosci Bull 2011, 27: 389–406.
Qi YB, Cheng HM, Wang Y, Chen Z. Revealing the precise role of calretinin neurons in epilepsy: We are on the way. Neurosci Bull 2022, 38: 209–222.
Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 2006, 140: 685–697.
Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol Ther 2019, 201: 77–93.
Lee KI, Lin JW, Su CC, Fang KM, Yang CY, Kuo CY, et al. Silica nanoparticles induce caspase-dependent apoptosis through reactive oxygen species-activated endoplasmic reticulum stress pathway in neuronal cells. Toxicol In Vitro 2020, 63: 104739.
Lichota A, Gwozdzinski L, Gwozdzinski K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur J Med Chem 2019, 176: 68–91.
Smilin Bell Aseervatham G, Abbirami E, Sivasudha T, Ruckmani K. Passiflora caerulea L Fruit extract and its metabolites ameliorate epileptic seizure, cognitive deficit and oxidative stress in pilocarpine-induced epileptic mice. Metab Brain Dis 2020, 35: 159–173.
Xie NC, Wang C, Wu CJ, Cheng X, Gao YL, Zhang HF, et al. Mdivi-1 protects epileptic hippocampal neurons from apoptosis via inhibiting oxidative stress and endoplasmic reticulum stress in vitro. Neurochem Res 2016, 41: 1335–1342.
Li MM, Li X, Cao ZL, Wu YT, Chen J, Gao J, et al. Mitochondria-targeting BODIPY-loaded micelles as novel class of photosensitizer for photodynamic therapy. Eur J Med Chem 2018, 157: 599–609.
Pierce JD, Gupte R, Thimmesch A, Shen QH, Hiebert JB, Brooks WM, et al. Ubiquinol treatment for TBI in male rats: Effects on mitochondrial integrity, injury severity, and neurometabolism. J Neurosci Res 2018, 96: 1080–1092.
Wang C, Xie NC, Wang YL, Li YL, Ge XJ, Wang ML. Role of the mitochondrial calcium uniporter in rat hippocampal neuronal death after pilocarpine-induced status epilepticus. Neurochem Res 2015, 40: 1739–1746.
Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res 2010, 88: 23–45.
Rowley S, Liang LP, Fulton R, Shimizu T, Day B, Patel M. Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy. Neurobiol Dis 2015, 75: 151–158.
Folbergrová J, Jesina P, Haugvicová R, Lisý V, Houstek J. Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid. Neurochem Int 2010, 56: 394–403.
Folbergrová J, Ješina P, Kubová H, Otáhal J. Effect of resveratrol on oxidative stress and mitochondrial dysfunction in immature brain during epileptogenesis. Mol Neurobiol 2018, 55: 7512–7522.
Ng CSH, Wan S, Arifi AA, Yim APC. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today 2006, 36: 205–214.
Chen K, Xu ZC, Liu YK, Wang Z, Li Y, Xu XF, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med 2017, 9: eaao6298.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481: 463–468.
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61: 1725–1738.
Erden Y, Tekin S, Sandal S, Onalan EE, Tektemur A, Kirbag S. Effects of central irisin administration on the uncoupling proteins in rat brain. Neurosci Lett 2016, 618: 6–13.
Zhang Y, Li R, Meng Y, Li SW, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63: 514–525.
Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010, 46: 2593–2604.
Wang Y, Xu CL, Xu ZH, Ji CH, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron 2017, 95: 92-105.e5.
Wang H, Zhao YT, Zhang SY, Dubielecka PM, Du JF, Yano N, et al. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol 2017, 232: 3775–3785.
Wang Z, Chen K, Han Y, Zhu H, Zhou XY, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol 2018, 72: 259–269.
Zhang WZ, Chang L, Zhang C, Zhang R, Li ZR, Chai BX, et al. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther 2015, 29: 121–127.
Abedpoor N, Taghian F, Ghaedi K, Niktab I, Safaeinejad Z, Rabiee F, et al. PPARγ/Pgc-1α-Fndc5 pathway up-regulation in gastrocnemius and heart muscle of exercised, branched chain amino acid diet fed mice. Nutr Metab (Lond) 2018, 15: 59.
Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 2002, 297: 79–83.
Wrann CD. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast 2015, 1: 55–61.
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 2013, 18: 649–659.
Dinda B, Dinda M, Kulsi G, Chakraborty A, Dinda S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur J Med Chem 2019, 169: 185–199.
Belviranli M, Okudan N, Kabak B, Erdoğan M, Karanfilci M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys Sportsmed 2016, 44: 290–296.
Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy. Curr Opin Drug Discov Devel 2006, 9: 580–586.
Xia DY, Huang X, Bi CF, Mao LL, Peng LJ, Qian HR. PGC-1α or FNDC5 is involved in modulating the effects of Aβ1-42 oligomers on suppressing the expression of BDNF, a beneficial factor for inhibiting neuronal apoptosis, Aβ deposition and cognitive decline of APP/PS1 Tg mice. Front Aging Neurosci 2017, 9: 65.
Falcicchia C, Paolone G, Emerich DF, Lovisari F, Bell WJ, Fradet T, et al. Seizure-suppressant and neuroprotective effects of encapsulated BDNF-producing cells in a rat model of temporal lobe epilepsy. Mol Ther Methods Clin Dev 2018, 9: 211–224.
Wang CF, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res 2010, 1336: 66–77.
Bonet ML, Mercader J, Palou A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie 2017, 134: 99–117.
Hoang T, Smith MD, Jelokhani-Niaraki M. Toward understanding the mechanism of ion transport activity of neuronal uncoupling proteins UCP2, UCP4, and UCP5. Biochemistry 2012, 51: 4004–4014.
Binienda ZK, Ali SF, Virmani A, Amato A, Salem N, Przybyla BD. Co-regulation of dopamine D1 receptor and uncoupling protein-2 expression in 3-nitropropionic acid-induced neurotoxicity: Neuroprotective role of L-carnitine. Neurosci Lett 2006, 410: 62–65.
Diano S, Matthews RT, Patrylo P, Yang LC, Beal MF, Barnstable CJ, et al. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: A mechanism for preconditioning. Endocrinology 2003, 144: 5014–5021.
Zhao BS, Sun LK, Jiang XR, Zhang Y, Kang JS, Meng H, et al. Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur J Pharmacol 2019, 845: 56–64.
Flori L, Testai L, Calderone V. The, “irisin system”: From biological roles to pharmacological and nutraceutical perspectives. Life Sci 2021, 267: 118954.
Zhu D, Wang HC, Zhang JL, Zhang XT, Xin C, Zhang FY, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 2015, 87: 138–147.
Ren YF, Qiu ML, Zhang J, Bi JB, Wang MZ, Hu LS, et al. Low serum irisin concentration is associated with poor outcomes in patients with acute pancreatitis, and irisin administration protects against experimental acute pancreatitis. Antioxid Redox Signal 2019, 31: 771–785.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972, 32: 281–294.
Liu T, Ma XR, Ouyang TX, Chen HP, Xiao Y, Huang YY, et al. Efficacy of 5-aminolevulinic acid-based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox Biol 2019, 20: 195–203.
Zhang MD, Cui YR, Zhu W, Yu J, Cheng Y, Wu XD, et al. Attenuation of the mutual elevation of iron accumulation and oxidative stress may contribute to the neuroprotective and anti-seizure effects of xenon in neonatal hypoxia-induced seizures. Free Radic Biol Med 2020, 161: 212–223.
Zhang YR, Zhang MD, Zhu W, Yu J, Wang QY, Zhang JJ, et al. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol 2020, 28: 101365.
Zhang YR, Zhang MD, Liu SH, Zhu W, Yu J, Cui YR, et al. Xenon exerts anti-seizure and neuroprotective effects in kainic acid-induced status epilepticus and neonatal hypoxia-induced seizure. Exp Neurol 2019, 322: 113054.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984, 11: 47–60.
Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006, 1: 848–858.
Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp 2011: 2920.
Pereira LO, Arteni NS, Petersen RC, da Rocha AP, Achaval M, Netto CA. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol Learn Mem 2007, 87: 101–108.
Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process 2012, 13: 93–110.
Mathiasen JR, DiCamillo A. Novel object recognition in the rat: A facile assay for cognitive function. Curr Protoc Pharmacol 2010, Chapter 5: Unit5.59.
Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 2017, 126: 55718.
Zhang R, Xue GZ, Wang SD, Zhang LH, Shi CJ, Xie X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer’s disease mouse model. J Alzheimers Dis 2012, 31: 801–812.
Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang LC, Beal MF, et al. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson’s disease. J Neurosci 2005, 25: 184–191.
Echtay KS. Mitochondrial uncoupling proteins—what is their physiological role? Free Radic Biol Med 2007, 43: 1351–1371.
Vaynman S, Ying Z, Gómez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res 2004, 76: 356–362.
Migliaccio V, Scudiero R, Sica R, Lionetti L, Putti R. Oxidative stress and mitochondrial uncoupling protein 2 expression in hepatic steatosis induced by exposure to xenobiotic DDE and high fat diet in male Wistar rats. PLoS One 2019, 14: e0215955.
Bannai H, Niwa F, Sherwood MW, Shrivastava AN, Arizono M, Miyamoto A, et al. Bidirectional control of synaptic GABAAR clustering by glutamate and calcium. Cell Rep 2015, 13: 2768–2780.
Yang XY, Tse MCL, Hu X, Jia WH, Du GH, Chan CB. Interaction of CREB and PGC-1α induces fibronectin type III domain-containing protein 5 expression in C2C12 myotubes. Cell Physiol Biochem 2018, 50: 1574–1584.
Bhatti J, Nascimento B, Akhtar U, Rhind SG, Tien H, Nathens A, et al. Systematic review of human and animal studies examining the efficacy and safety of N-Acetylcysteine (NAC) and N-Acetylcysteine amide (NACA) in traumatic brain injury: Impact on neurofunctional outcome and biomarkers of oxidative stress and inflammation. Front Neurol 2017, 8: 744.
Askari H, Rajani SF, Poorebrahim M, Haghi-Aminjan H, Raeis-Abdollahi E, Abdollahi M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol Res 2018, 129: 44–55.
Batirel S, Bozaykut P, Mutlu Altundag E, Kartal Ozer N, Mantzoros CS. The effect of Irisin on antioxidant system in liver. Free Radic Biol Med 2014, 75(Suppl 1): S16.
Lu JY, Xiang GD, Liu M, Mei W, Xiang L, Dong J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243: 438–448.
Pearson-Smith JN, Patel M. Metabolic dysfunction and oxidative stress in epilepsy. Int J Mol Sci 2017, 18: E2365.
Elhady M, Youness ER, Gafar HS, Abdel Aziz A, Mostafa RSI. Circulating irisin and chemerin levels as predictors of seizure control in children with idiopathic epilepsy. Neurol Sci 2018, 39: 1453–1458.
Asadi Y, Gorjipour F, Behrouzifar S, Vakili A. Irisin peptide protects brain against ischemic injury through reducing apoptosis and enhancing BDNF in a rodent model of stroke. Neurochem Res 2018, 43: 1549–1560.
Greenberg ME, Xu BJ, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci 2009, 29: 12764–12767.
Wu BW, Wu MS, Guo JD. Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease. J Cell Physiol 2018, 233: 5281–5292.
Lin TW, Harward SC, Huang YZ, McNamara JO. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology 2020, 167: 107734.
Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 2014, 220: 223–250.
Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol 2010, 25: 237–258.
Kang DH, Choi BY, Lee SH, Kho AR, Jeong JH, Hong DK, et al. Effects of cerebrolysin on hippocampal neuronal death after pilocarpine-induced seizure. Front Neurosci 2020, 14: 568813.
Nieto-Gonzalez JL, Jensen K. BDNF depresses excitability of parvalbumin-positive interneurons through an M-like current in rat dentate gyrus. PLoS One 2013, 8: e67318.
Osehobo P, Adams B, Sazgar M, Xu Y, Racine RJ, Fahnestock M. Brain-derived neurotrophic factor infusion delays amygdala and perforant path kindling without affecting paired-pulse measures of neuronal inhibition in adult rats. Neuroscience 1999, 92: 1367–1375.
LaFrance WC Jr, Leaver K, Stopa EG, Papandonatos GD, Blum AS. Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 2010, 75: 1285–1291.
Safari F, Anvari Z, Moshtaghioun S, Javan M, Bayat G, Forosh SS, et al. Differential expression of cardiac uncoupling proteins 2 and 3 in response to myocardial ischemia-reperfusion in rats. Life Sci 2014, 98: 68–74.
Safari F, Bayat G, Shekarforoush S, Hekmatimoghaddam S, Anvari Z, Moghadam MF, et al. Expressional profile of cardiac uncoupling protein-2 following myocardial ischemia reperfusion in losartan- and ramiprilat-treated rats. J Renin Angiotensin Aldosterone Syst 2014, 15: 209–217.
Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): A protective stress signal in neuronal injury. Biochem Pharmacol 2002, 64: 363–367.
Guo XP, Zhang MM, Gao Y, Cao GZ, Lu D, Li WJ. Quantitative multi-omics analysis of the effects of mitochondrial dysfunction on lipid metabolism in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 2020, 104: 1211–1226.
Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology 2003, 144: 2757–2760.
Gupta RC, Milatovic D, Dettbarn WD. Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: Protection by antioxidants. Neurotoxicology 2001, 22: 271–282.
Gupta RC, Milatovic D, Dettbarn WD. Nitric oxide modulates high-energy phosphates in brain regions of rats intoxicated with diisopropylphosphorofluoridate or carbofuran: Prevention by N-tert-butyl-alpha-phenylnitrone or vitamin E. Arch Toxicol 2001, 75: 346–356.
Chan SHH, Wu CWJ, Chang AYW, Hsu KS, Chan JYH. Transcriptional upregulation of brain-derived neurotrophic factor in rostral ventrolateral medulla by angiotensin II: Significance in superoxide homeostasis and neural regulation of arterial pressure. Circ Res 2010, 107: 1127–1139.
Bumanglag AV, Sloviter RS. No latency to dentate granule cell epileptogenesis in experimental temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2018, 59: 2019–2034.
Wu J, Vogel T, Gao X, Lin B, Kulwin C, Chen JH. Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury. Sci Rep 2018, 8: 4935.
Tse K, Hammond D, Simpson D, Beynon RJ, Beamer E, Tymianski M, et al. The impact of postsynaptic density 95 blocking peptide (Tat-NR2B9c) and an iNOS inhibitor (1400W) on proteomic profile of the hippocampus in C57BL/6J mouse model of kainate-induced epileptogenesis. J Neurosci Res 2019, 97: 1378–1392.
Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, et al. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 2016, 351: aaa5694.
Cheng HM, Wang Y, Chen JZ, Chen Z. The piriform cortex in epilepsy: What we learn from the kindling model. Exp Neurol 2020, 324: 113137.
Jin Z, Guo PP, Li XY, Ke JJ, Wang YL, Wu HS. Neuroprotective effects of irisin against cerebral ischemia/reperfusion injury via Notch signaling pathway. Biomed Pharmacother 2019, 120: 109452.
Chen C, Li H, Ding F, Yang LL, Huang PY, Wang S, et al. Alterations in the hippocampal-thalamic pathway underlying secondarily generalized tonic-clonic seizures in mesial temporal lobe epilepsy: A diffusion tensor imaging study. Epilepsia 2019, 60: 121–130.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81573412 and 81803546), Key Research and Development Plan of Shandong Province (2018GSF121004), and Yantai Science and Technology Development Plan (2019xdhz098). We would like to thank Editage for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J., Cheng, Y., Cui, Y. et al. Anti-Seizure and Neuronal Protective Effects of Irisin in Kainic Acid-Induced Chronic Epilepsy Model with Spontaneous Seizures. Neurosci. Bull. 38, 1347–1364 (2022). https://doi.org/10.1007/s12264-022-00914-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00914-w