Abstract
Objective
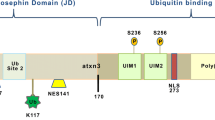
Machado-Joseph disease (MJD), also known as spinocerebellar ataxia type 3 (SCA3), is a dominant neurodegenerative disorder caused by an expansion of the polyglutamine (polyQ) tract in MJD-1 gene product, ataxin-3 (AT3). This disease is characterized by the formation of intraneuronal inclusions, but the mechanism underlying their formation is still poorly understood. The present study is to explore the relationship between wild type (WT) AT3 and polyQ expanded AT3.
Methods
Mouse neuroblastoma (N2a) cells or HEK293 cells were co-transfected with WT AT3 and different truncated forms of expanded AT3. The expressions of WT AT3 and the truncated forms of expanded AT3 were detected by Western blotting, and observed by an inverted fluorescent microscope. The interactions between AT3 and different truncated forms of expanded AT3 were detected by immunoprecipitation and GST pull-down assays.
Results
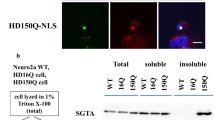
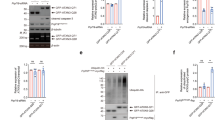
Using fluorescent microscope, we observed that the truncated forms of expanded AT3 aggregate in transfected cells, and the full-length WT AT3 is recruited onto the aggregates. However, no aggregates were observed in cells transfected with the truncated forms of WT AT3. Immunoprecipitation and GST pull-down analyses indicate that WT AT3 interacts with the truncated AT3 in a polyQ length-dependent manner.
Conclusion
WT AT3 deposits in the aggregation that was formed by polyQ expanded AT3, which suggests that the formation of AT3 aggregation may affect the normal function of WT AT3 and increase polyQ protein toxicity in MJD.
摘要
目的
脊髓小脑共济失调III型(spinocerebellar ataxia type 3, SCA3)/马查多-约瑟夫病(Machado-Joseph disease, MJD)是MJD-1基因中编码谷氨酰胺的密码子CAG的数量非正常扩增引起的一种多聚谷氨酰胺疾病, 也是神经退行性疾病的一种。 该病的主要病理特征为突变的ataxin-3在患者易感脑区的神经元胞核内聚集形成核内包涵体, 但其致病机制和突变蛋白在核内聚集的机制仍不清楚。 本研究是为了探讨突变型ataxin-3的病理学特性以及其聚集特性。
方法
将野生型ataxin-3及不同多聚谷氨酰胺长度的突变型ataxin-3片段共转染到人胚胎肾细胞(HEK293 cells)和鼠成神经母细胞(neuroblastoma cells)中, 用荧光显微镜观察。 共转染48小时后, 收集在细胞中表达的蛋白, 用免疫印迹、 免疫共沉淀和GST-pull down实验检测蛋白的表达及结合。
结果
用荧光显微镜观察到突变型ataxin-3片段在细胞内形成聚集, 其聚集体上募集有野生型全长ataxin-3; 突变型和野生型ataxin-3在细胞内聚集体上有共定位现象。 野生型ataxin-3片段并不形成聚集体, 与野生型全长ataxin-3在细胞内无聚集现象发生。 免疫共沉淀技术及GST-pulldown实验显示, 突变型ataxin-3片段与野生型全长ataxin-3存在相互作用, 且该作用强度呈现出多聚谷氨酰胺长度依赖性。
结论
结果提示, 野生型ataxin-3通过与突变型ataxin-3片段的相互作用沉积于突变片段形成的聚集体中, 这可能影响野生型ataxin-3的正常功能, 进而可能对脊髓小脑共济失调III型(SCA3)/马查多-约瑟夫病(MJD)的发病产生影响。
Similar content being viewed by others
References
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet 1994, 8: 221–228.
Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol 2002, 3: 246–255.
Durr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, et al. Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol 1996, 39: 490–499.
Nishiyama K, Murayama S, Goto J, Watanabe M, Hashida H, Katayama S, et al. Regional and cellular expression of the Machado-Joseph disease gene in brains of normal and affected individuals. Ann Neurol 1996, 40: 776–781.
Sudarsky L, Coutinho P. Machado-Joseph disease. Clin Neurosci 1995, 3: 17–22.
Paulson HL, Das SS, Crino PB, Perez MK, Patel SC, Gotsdiner D, et al. Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain. Ann Neurol 1997, 41: 453–462.
Li F, Macfarlan T, Pittman RN, Chakravarti D. Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities. J Biol Chem 2002, 277: 45004–45012.
Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet 2003, 12: 3195–3205.
Evert BO, Vogt IR, Vieira-Saecker AM, Ozimek L, de Vos RA, Brunt ER, et al. Gene expression profiling in ataxin-3 expressing cell lines reveals distinct effects of normal and mutant ataxin-3. J Neuropathol Exp Neurol 2003, 62: 1006–1018.
Zoghbi HY, Orr HT. Polyglutamine diseases: protein cleavage and aggregation. Curr Opin Neurobiol 1999, 9: 566–570.
Wanker EE. Protein aggregation and pathogenesis of Huntington’s disease: mechanisms and correlations. Biol Chem 2000, 381: 937–942.
Wanker EE. Protein aggregation in Huntington’s and Parkinson’s disease: implications for therapy. Mol Med Today 2000, 6: 387–391.
Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med 2004, 10Suppl: S10–S17.
Goti D, Katzen SM, Mez J, Kurtis N, Kiluk J, Ben-Haiem L, et al. A mutant ataxin-3 putative-cleavage fragment in brains of Machado-Joseph disease patients and transgenic mice is cytotoxic above a critical concentration. J Neurosci 2004, 24: 10266–10279.
Haacke A, Broadley SA, Boteva R, Tzvetkov N, Hartl FU, Breuer P. Proteolytic cleavage of polyglutamine-expanded ataxin-3 is critical for aggregation and sequestration of non-expanded ataxin-3. Hum Mol Genet 2006, 15: 555–568.
Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N. Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B. Hum Mol Genet 2000, 9: 1795–1803.
Fei E, Jia N, Zhang T, Ma X, Wang H, Liu C, et al. Phosphorylation of ataxin-3 by glycogen synthase kinase 3beta at serine 256 regulates the aggregation of ataxin-3. Biochem Biophys Res Commun 2007, 357: 487–492.
Wang G, Ide K, Nukina N, Goto J, Ichikawa Y, Uchida K, et al. Machado-Joseph disease gene product identified in lymphocytes and brain. Biochem Biophys Res Commun 1997, 233: 476–479.
Heiser V, Scherzinger E, Boeddrich A, Nordhoff E, Lurz R, Schugardt N, et al. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington’s disease therapy. Proc Natl Acad Sci U S A 2000, 97: 6739–6744.
Ellisdon AM, Thomas B, Bottomley SP. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J Biol Chem 2006, 281: 16888–16896.
Tanaka M, Machida Y, Nishikawa Y, Akagi T, Hashikawa T, Fujisawa T, et al. Expansion of polyglutamine induces the formation of quasi-aggregate in the early stage of protein fibrillization. J Biol Chem 2003, 278: 34717–34724.
Cowan KJ, Diamond MI, Welch WJ. Polyglutamine protein aggregation and toxicity are linked to the cellular stress response. Hum Mol Genet 2003, 12: 1377–1391.
Iuchi S, Hoffner G, Verbeke P, Djian P, Green H. Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine. Proc Natl Acad Sci U S A 2003, 100: 2409–2414.
Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet 2002, 11: 2905–2917.
Tam S, Geller R, Spiess C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol 2006, 8: 1155–1162.
Kitamura A, Kubota H, Pack CG, Matsumoto G, Hirayama S, Takahashi Y, et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol 2006, 8: 1163–1170.
Burnett BG, Pittman RN. The polyglutamine neurodegenerative protein ataxin 3 regulates aggresome formation. Proc Natl Acad Sci USA 2005, 102: 4330–4335.
Bevivino AE, Loll PJ. An expanded glutamine repeat destabilizes native ataxin-3 structure and mediates formation of parallel beta-fibrils. Proc Natl Acad Sci USA 2001, 98: 11955–11960.
Schaffar G, Breuer P, Boteva R, Behrends C, Tzvetkov N, Strippel N, et al. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell 2004, 15: 95–105.
Gusella JF, MacDonald ME. Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci 2000, 1: 109–115.
Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, Das SS, et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 1997, 19: 333–344.
Evert BO, Araujo J, Vieira-Saecker AM, de Vos RA, Harendza S, Klockgether T, et al. Ataxin-3 represses transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation. J Neurosci 2006, 26: 11474–11486.
Warrick JM, Morabito LM, Bilen J, Gordesky-Gold B, Faust LZ, Paulson HL, et al. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell 2005, 18: 37–48.
Busch A, Engemann S, Lurz R, Okazawa H, Lehrach H, Wanker EE. Mutant huntingtin promotes the fibrillogenesis of wild-type huntingtin: a potential mechanism for loss of huntingtin function huntington’s disease.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jia, NL., Fei, EK., Ying, Z. et al. PolyQ-expanded ataxin-3 interacts with full-length ataxin-3 in a polyQ length-dependent manner. Neurosci. Bull. 24, 201–208 (2008). https://doi.org/10.1007/s12264-008-0326-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-008-0326-9