Abstract
Objective
To search novel genes or pathways involved in the recovery process after restraint stress in rats.
Methods
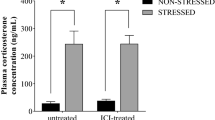
We compared the hypothalamus transcriptional profiles of two different recovery patterns (fast recovery vs slow recovery) from restraint stress in rats using oligonucleotide microarray, the recovery pattern was determined by the decrement of plasma adrenocorticotropic-hormone (ACTH) and corticosterone levels during one hour recovery period after stress. A real-time quantitative RT-PCR was applied to validate the differential expressed genes.
Results
Analysis of the microarray data showed that most of genes were not differentially expressed between fast recovery group and slow recovery group. Among the differentially expressed genes we found that talin, together with serine/threonine protein phosphatase PP1-beta catalytic subunit (PP-1B) and integrin α-6 precursor (VLA-6) genes, were at least 1.5 fold up-regulated in the fast recovery group, while junctional adhesion molecule 1 (F11r) was 1.5 fold down-regulated in the fast recovery group.
Conclusion
The results implied that integrin signaling pathway may be involved in the recovery from restraint stress in rats. The present study provided a global overview of hypothalamus transcriptional profiles during the process of recovery from the restraint stress in rats. The integrin signaling pathway seems to be involved in the recovery process, which deserves further study to clarify the integrin-mediated recovery mechanism after restraint stress.
摘要
目的
筛选与束缚应激大鼠应激反应恢复速度相关的基因, 探讨应激反应个体恢复的分子机制。
方法
观察大鼠2 小时束缚应激后血浆促肾上腺皮质激素(adrenocorticotropic-hormone, ACTH)及皮质酮的变化规律, 根据应激结束后1小时的血浆ACTH及皮质酮下降程度将大鼠分为快速恢复组与慢速恢复组。采用微阵列技术检测快速恢复组与慢速恢复组下丘脑组织基因表达的差异, 用实时逆转录-聚合酶链反应验证部分基因的表达差异。
结果
基因芯片分析结果显示: 大部分基因在两组间并无差异表达, 在11 个差异表达的基因中, 快速恢复组中参与整合素信号通路的踝蛋白(talin)、整合素α6和丝/ 苏氨酸蛋白磷酸酶PP1β催化亚基(serine/threonine protein phosphatase PP1-beta catalytic subunit, PP1B)基因有1.5 倍上调, 而结合粘附分子1(junctional adhesion molecule 1, F11r)基因有1.5 倍下调。实时逆转录-聚合酶链反应结果与芯片分析结果一致。
结论
本研究构建了束缚应激大鼠恢复过程的下丘脑基因表达谱, 结果提示整合素信号通路可能参与应激的恢复过程。
Similar content being viewed by others
References
Guimont S, Wynne-Edwards K. Individual variation in cortisol responses to acute “on-back” restraint in an outbred hamster. Horm Behav 2006, 50: 252–260.
Roy MP, Kirschbaum C, Steptoe A. Psychological, cardiovascular, and metabolic correlates of individual differences in cort isolstres s recovery in young men. Psychoneuroendocrinology 2001, 26: 375–391.
de Kloet ER. Hormones, brain and stress. Endocr Reg 2003, 37: 51–68.
Lundberg U. Stress hormones in health and illness: The roles of work and gender. Psychoneuroendocrinology 2005, 30: 1017–1021.
de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005, 6: 463–475.
Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: conceptual siblings separated at birth? J Psychosom Res 1997, 42: 117–135.
Garcia A, Armario A. Individual differences in the recovery of the hypothalamic-pituitary-adrenal axis after termination of exposure to a severe stressor in outbred male Sprague-Dawley rats. Psychoneuroendocrinology 2001, 26: 363–374.
Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology 2000, 72: 114–125.
Lee HC, Chang DE, Yeom M, Kim GH, Choi KD, Shim I, et al. Gene expression profiling in hypothalamus of immobilization-stressed mouse using cDNA microarray. Brain Res Mol Brain Res 2005, 135: 293–300.
Mizuno S, Kato K, Asai S, Takahashi Y, Nagata T, Komuro S, et al. Gene expression analysis in the stomachs of water immersion-restraint stress rats using high-density oligonucleotide array. J Gastroenterol Hepatol 2004, 19: 1264–1269.
Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 2005, 289: E823–828.
Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 2003, 23: 5607–5616.
Bueno Filho JS, Gilmour SG, Rosa GJ. Design of microarray experiments for genetical genomics studies. Genetics 2006, 174: 45–57.
Cui X, Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol 2003, 4: 210.
Cui X, Hwang JT, Qiu J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics 2005, 6: 59–75.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25: 402–408.
Keller-Wood ME, Shinsako J, Keil LC, Dallman MF. Insulin-induced hypoglycemia in conscious dogs: I. dose-related pituitary and adrenal responses. Endocrinology 1981, 109: 818–824.
Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992, 267: 1244–1252.
Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress 2003, 6: 87–100.
Nayal A, Webb DJ, Horwitz AF. Talin: an emerging focal point of adhesion dynamics. Curr Opin Cell Biol 2004, 16: 94–98.
Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science 2003, 302: 103–106.
Shenolikar S. Protein serine/threonine phosphatases-new avenues for cell regulation. Annu Rev Cell Biol 1994, 10: 55–86.
Broustas CG, Grammatikakis N, Eto M, Dent P, Brautigan DL, Kasid U. Phosphorylation of the myosin-binding mubunit of myosin phosphatase by Raf-1 and inhibition of phosphatase activity. J Biol Chem 2002, 277: 3053–3059.
Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci 2004, 12: 917–930.
Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 2004, 279: 16254–16262.
Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem 2005, 280: 11665–11674.
Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 2002, 2: 151–158.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, YZ., Guo, SY., Yin, QZ. et al. Possible involvement of integrin signaling pathway in the process of recovery from restraint stress in rats. Neurosci. Bull. 23, 229–235 (2007). https://doi.org/10.1007/s12264-007-0034-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-007-0034-x
Keywords
- physical restraint
- adrenocorticotropic hormone
- corticosterone
- post-stress recovery
- oligonucleotide microarray