Abstract
Hereditary hemochromatosis is an iron overload disorder most commonly caused by a defect in the HFE gene. While the genetic defect is highly prevalent, the majority of individuals do not develop clinically significant iron overload, suggesting the importance of genetic modifiers. Murine hfe knockout models have demonstrated that strain background has a strong effect on the severity of iron loading. We noted that hepatic iron loading in hfe−/− mice occurs primarily over the first postnatal weeks (loading phase) followed by a timeframe of relatively static iron concentrations (plateau phase). We thus evaluated the effects of background strain and of age on hepatic gene expression in Hfe knockout mice (hfe−/−). Hepatic gene expression profiles were examined using cDNA microarrays in 4- and 8-week-old hfe−/− and wild-type mice on two different genetic backgrounds, C57BL/6J (C57) and AKR/J (AKR). Genes differentially regulated in all hfe−/− mice groups, compared with wild-type mice, including those involved in cell survival, stress and damage responses and lipid metabolism. AKR strain-specific changes in lipid metabolism genes and C57 strain-specific changes in cell adhesion and extracellular matrix protein genes were detected in hfe−/− mice. Mouse strain and age are each significantly associated with hepatic gene expression profiles in hfe−/− mice. These affects may underlie or reflect differences in iron loading in these mice.
Similar content being viewed by others
Introduction
Hereditary hemochromatosis (HH), caused by defective HFE function, is an iron overload disorder that affects one in 400 people with a carrier frequency of one in 10 individuals of Northern European descent (Feder et al. 1996; Fleming and Sly 2002) . Wild-type HFE forms a complex with β2-microglobulin in the endothelium reticulum (ER) and translocates to the cell surface (Feder et al. 1998; Gross et al. 1998; Roy et al. 1999). A current model suggests that the HFE protein acts by forming a complex with transferrin receptors 1 or 2 and influencing hepatocellular signaling in the regulation of the hepcidin gene (Feder et al. 1998; Gross et al. 1998; Roy et al. 1999). A point mutation in HFE (845G → A), which accounts for the vast majority cases of HH, results in the amino acid substitution of C282Y (Feder et al. 1996). The C282Y mutation disrupts a disulfide bond required for the interaction with β2-microglobulin, and the heterodimer fails to move to the plasma membrane (Feder et al. 1997; Waheed et al. 1997). Heterozygotes for HFE gene are demonstrated to have elevated serum levels of body iron store indicators such as serum ferritin and transferrin saturation (Bulaj et al. 1996). More than 90 % of patients with HH ascertained by clinical manifestations of liver disease are homozygous for the C282Y mutation in the HFE gene (Bacon et al. 1999). However, population-wide surveys demonstrate that fewer than 10 % of individuals with homozygous C282Y have clinical manifestations (Mura et al. 2001; Beutler et al. 2002; Sanchez et al. 2003). The low penetrance in HFE-associated hemochromatosis suggests that other factors such as genetic variation in other genes likely underlies differences in iron loading in people with this mutation (Rochette et al. 2010).
Mouse models for HH that were generated by knocking out the Hfe gene in C57 and AKR backgrounds showed strain effects on the levels of hepatic iron loading in hfe−/− mice (Fleming et al. 2001). Ten-week-old hfe−/− mice from the AKR background showed two to three times higher hepatic iron accumulation as compared to age-matched hfe−/− mice from a C57 background (Fleming et al. 2001). Differences in hepatic iron levels between hfe−/− mice and wild-type mice start to increase after 2 weeks of age in both strains (Fleming et al. 2001). Moreover, to reach complete saturation of serum transferrin, C57 mice needed a 10 times higher oral dose of carbonyl iron as compared to AKR mice (Leboeuf et al. 1995).
We aimed to characterize the effects of strain and/or age-associated iron loading stages on gene expression profiles in hfe−/− mice. We carried out cDNA microarray studies using hfe−/− 8-week-old C57 mice, 4-week-old AKR mice, 8-week-old AKR and wild-type mice. We first investigated whether there were differences in several selected genes known to be involved in iron metabolism by real-time PCR. Subsequently, we screened a set of ~11,000 genes by expression profiling using microarrays to identify differentially expressed genes that may play a role in strain-specific variation in iron loading in hfe−/− mice.
Materials and methods
Mice
hfe−/− male mice and wild-type AKR and C57 male mice were fed a standard chow (270 mg/kg of iron) ad libitum. Four-week-old and 8-week-old AKR mice and 8-week-old C57 mice were killed (8-week-old C57 group). Prior to killing, mice were fasted overnight and euthanized before liver tissue samples were collected. The studied mouse population consisted of 6–8 of hfe−/− mice. The animal protocols were approved by the Animal Care and Use Committees of Saint Louis University.
Reverse transcription (RT) for microarray
We used 12.5 μg of pooled total RNA samples isolated from the liver of 6 to 8 hfe−/− or wild-type mice in each group to synthesize cDNA. Only RNA samples with the ratio of A260 to A280 higher than 2.0 and the presence of two clear bands indicating intact 18S rRNA and 28S rRNA when run on a RNA gel electrophoresis were used for the study. Reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) primed by Oligo dT in the presence of aminoallyl dUTP. The resulting amino-modified cDNA was labeled using Cy3 or Cy5 fluorescent dyes (Amersham Biosciences, Piscataway, NJ).
BMAP microarray slide
The cDNA microarrays were produced from Brain Mouse Anatomy Project (BMAP) mouse brain UniGene set (Open Biosystems, Huntsville, AL). cDNAs were amplified using primers adjacent to cloning sites by polymerase chain reaction (PCR). Purified PCR products were spotted onto poly-lysine-coated slides using a custom built arrayer at the College of Natural Resources Genomics Facility at the University of California, Berkeley.
Microarray hybridization and image acquisition
We performed eight hybridizations for each experimental group (4- and 8-week-old AKR groups and 8-week-old C57 group) to compare hepatic expression profiles from hfe−/− mice to those of wild-type mice. The Cy3- and Cy5- labeled cDNAs were mixed prior to hybridization to cDNA microarrays. Hybridization was performed at 65 °C for 16–20 h. Microarray images were obtained by scanning slides using an ArrayWoRx Biochip Reader (Applied Precision, Issaquah, WA). Images were quantified using GenePix 3.01 (Molecular Devices, Sunnyvale, CA) to obtain an average hybridization signal intensity for each spot and the background of the surrounding area.
Data analysis
Spot intensity values for each array were analyzed using our exploratory differential gene expression analysis algorithm (Loguinov et al. 2004). Spot intensities were normalized by nonparametric regression smoothing (super smoother, supsmu, also known as lowess). Using this method, data were grouped into subsets of equal size (print tip groups) and then regression smoothers were applied to the median absolute residual against the median of log2-transformed Cy3 for each box plot group. Log2-transformed normalized ratios were used for subsequent data analysis to identify candidate genes for differential expression. A general α-outlier model for residuals was adapted, and smoothed simultaneous tolerance intervals (STIs) were applied to account for heteroscedasticity of residual variance. We selected candidate genes with a p value lower than 0.001 that were either up- or down-regulated in at least four out of eight hybridizations. Candidate genes were analyzed for their association with biological functions and/or diseases by Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, CA).
Reverse transcription (RT) and real-time quantitative polymerase chain reaction (RT-qPCR)
2 μg of DNaseI-treated total RNA that was isolated using Trizol reagent (Invitrogen) was reverse transcribed at 42 °C for 1 h using random primer and SuperscriptII reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. The resulting cDNA was diluted ten times in autoclaved DEPC water and subjected to qPCR. qPCR was performed with the ABI Prism 7700 and SYBR Green Reagents (Applied Biosystems, Foster City, CA). Two-step qPCR protocol was used with 40 cycles of 95 °C for 15 s and 60 °C for 60 s. PCR efficiency was confirmed by dilution curves from serially diluted cDNA samples. The relative abundances of mRNAs were obtained based on the 2−∆∆Ct method calculation (Beutler et al. 2002) and normalized to the amounts of 18S ribosomal RNA. The used primers are following: 18S ribosomal RNA F5′-GTAACCCGTTGAACCCCATT-3′, R 5′-CCATCCAATCGGTAGTAGCG-3′; Transferrin receptor 1 (Tfr1) F5′-TCATGAGGGAAATCAATGATCGTA-3′, R 5′-GCCCCAGAAGATATGTCGGAA-3′; Transferrin receptor 2 (Tfr2) F5′-CCCATGGACAGCAGTGCATAT-3′, R 5′-CGTGTGCAGGAATGGGTACA-3′; Ferritin heavy chain (Fth) F5′-TGCACAAACTGGCTACTGACAA-3′, R 5′-GGTCACCCAGTTCTTTAATGGATT-3′; Ferroportin (Fpn1or Slc40a1) F 5′-GGGAGCCATCATTGGTGACT-3′, R 5′-GATGATTCCGCAGAGGATGAC-3′; Hepcidin 1 (Hamp1) F 5′-GGCAGACATTGCGATACCAA-3′, R 5′-TGGCTCTAGGCTATGTTTTGCA-3′; Hepcidin 2 (Hamp2) F 5′-GCAGACATTGCGATCCCAA-3′, R 5′-CTCTCTATTCTTCACAACAGATACCACA-3′.
Results
Regulation of iron gene expression is affected by strains and iron loading status in hfe−/− mice
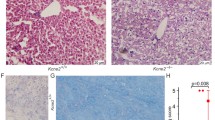
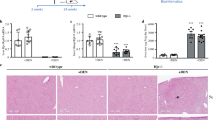
We observed that AKR mice accumulate iron during the first 8 weeks of life (loading phase), followed by relatively constant iron content (plateau phase) afterward (Fig. 1). The AKR wild-type mice demonstrate more hepatocellular iron than the C57 wild-type mice at all ages (Fig. 1). We thus compared hepatic gene expression profiles in AKR hfe−/− mice during “loading” (4 weeks old) and “plateau” (8 weeks old) phases with age-matched wild-type AKR mice. We also compared AKR hfe−/− mice with C57 hfe−/− mice at 8 weeks of age. We initially analyzed mRNA levels of selected genes: transferrin receptor (Tfr1 and Tfr2), hepcidin (Hamp1 and Hamp2), and Ferroportin 1 (Slc40a1) using pooled RNA samples by RT-qPCR (Fig. 2). Iron loading phase (4 week age) AKR hfe−/− mice showed a significant reduction in Tfr1, Hamp1, Hamp2, and Slc40a1 mRNA levels, but no changes in Tfr2 mRNAs, compared to age-matched WT mice (Fig. 2). Iron plateau phase (8 week age) AKR hfe−/− mice displayed a significant decreases in Tfr2, Hamp1, and Hamp2 mRNAs, an increase in Slc40a1 mRNA, and no changes in Tfr1 (Fig. 2) compared with wild-type mice of same age. Iron plateau phase (8 week age) C57 hfe−/− mice had elevated mRNA levels of Tfr1, Hamp1, and Hamp2, but decreased levels of Slc40a1, and no changes in Tfr2 as compared to wild-type C57 mice.
Relative mRNA levels of iron-related genes in AKR and C57 hfe−/− mice compared to wild-type mice. RNA pooled from five to eight individuals in each mice group was used for quantitative real-time PCR using SYBR green. Expression values were normalized to the 18SrRNA. Transcript levels of Tfr1 (a), Tfr2 (b), Hamp1 (c), Hamp2 (d), and Slc40a1 (e) from hfe−/− mice were compared to those from wild-type mice. The results are expressed as mean ± SD. # Comparisons of the expression levels of genes between KO and WT mice were performed by Student’s t test, p < 0.05; comparisons of the expression ratio of KO to WT between two groups were performed by Student’s t test, *p < 0.05, **p < 0.01, ***p < 0.005
Differences in strains and iron loading status cause few hepatic genes to be commonly influenced by hfe−/−
We expanded the repertoire of analyzed genes to include all those represented in an 11K BMAP microarray. We identified 26, 51, and 79 genes to be differentially expressed in the 4-week AKR, 8-week AKR, and 8-week C57BL6/J hfe−/− mice, respectively, as compared to WT controls (Supplementary Table 1–3). Interestingly, no gene was commonly dysregulated in all hfe−/− groups (Fig. 3). Comparisons between two groups either by strain or by iron plateau phase revealed that most differentially regulated genes were specific to each group, and few genes were commonly regulated (Fig. 3). The iron loading phase (4 weeks) and the iron plateau phase (8 week age) of AKR hfe−/− mice had only two genes commonly up-regulated, which were Zdhhc14 (zinc finger, DHHC domain containing 14) and Gabra6 (GABA-A receptor, subunit alpha6) (Table 1). AKR and C57 hfe−/− strains in the iron plateau phase shared one unidentified EST gene (Mm.396822) that was regulated in a similar fashion (Table 1). Eight-week-old AKR hfe−/− mice have higher hepatic iron concentrations than do the other hfe−/− mouse groups (8-week-old C57 hfe−/− and 4-week-old AKR hfe−/− mouse groups) but demonstrated differential regulation of only Nfatc1 (nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1) (Table 1). The lack of a common differential gene expression profile in the hfe−/− mouse groups demonstrates the profound influences of strain and age on the consequences of loss of Hfe.
Biological functional groups of cell survival, stress response, and lipid metabolism were disturbed in all hfe−/− mouse groups
We categorized the differentially expressed genes in each hfe−/− mouse group based on the known or predicted biological function of the protein product using Ingenuity Pathways Analysis. Functional groups of cell survival/growth, stress/damage response, and lipid metabolism were affected in all three hfe−/− mouse groups, representing 31, 38, and 25 % of the total number of genes identified in 4-week-old AKR, 8-week-old AKR, and 8-week-old C57 groups, respectively (Fig. 4). Although genes involved in biological processes were different among the hfe−/− mouse groups, cell survival/growth and stress/damage response pathways were up-regulated in hfe−/− mouse groups (Table 1). AKR hfe−/− mouse groups had common disturbances in a heme-related genes, representing 8 % of total genes in the iron loading phase (4 week age) group and 4 % of the total genes in the iron plateau phase (8 week age) (Fig. 5). Genes participating in cellular signaling were dysregulated in both iron plateau phases of AKR (4 % of the total) and C57 (3 % of the total) hfe−/− mouse groups (Fig. 5).
Strains and iron loading phases affect different biological functional groups in hfe−/− mouse groups
Analysis of differences in iron loading status of AKR hfe−/− mouse groups revealed that groups of genes related to drug metabolism, immune response, and extracellular matrix are dysregulated specifically in the iron loading phase of the AKR hfe−/− mouse group. Dysregulation of genes in transport, fibrosis, and signaling is specific to the iron plateau phase of the AKR hfe−/− mouse group (Fig. 4). When the iron plateau phases of AKR and C57 hfe−/− groups were compared, the AKR strain-specific dysregulation was seen in genes in transport, fibrosis, and heme-related functions. Immune response, extracellular matrix, cell adhesion, vesicle trafficking, RNA splicing, metabolism, and calcium regulation were only dysregulated in the C57 strain (Fig. 4).
Discussion
There are considerable differences in clinical findings in patients with HH (Beutler et al. 2002; Olynyk et al. 1999); for example, body iron indices range widely from 253 to 9,530 μg/L of serum ferritin and 1,926 to 50,887 μg/g of liver iron (Adams et al. 2006a). Some HH patients develop liver fibrosis (Olynyk et al. 2005; Adams et al. 2006b), cirrhosis (Asberg et al. 2001), hepatic steatosis (Adams et al. 2006a), steatohepatitis (Adams et al. 2006a), and coronary heart disease (Rasmussen et al. 2001), while most do not. In addition, biochemical parameters for iron loading including serum ferritin and/or transferrin saturation are not always elevated in C282Y homozygotes (Asberg et al. 2001; Beutler et al. 2002; Olynyk et al. 1999). Different family members who were homozygous for C282Y mutations demonstrated different clinical outcomes, suggesting that the existence of modifier genes to influence manifestation of the disease phenotype of HFE (Bulaj et al. 2000). Other genetic factors could include another mutation in the HFE gene and/or mutations in genes known to be involved in iron metabolism causing non-HFE hemochromatosis (Whitfield et al. 2000; Lee et al. 2004; Roetto et al. 2003; Camaschella et al. 2000). The risk of iron loading was higher when C282Y and H63D mutations co-exist in HFE gene as a compound heterozygote (Beutler 2006; Aranda et al. 2010). A gender effect on iron loading phenotypes in HFE mutations was noted with male gender having higher risks (Aranda et al. 2010). Epigenetic factors and environmental factors such as dietary iron and alcohol also could play a role in determining the incidence of the disease. Alcohol consumption increased serum ferritin levels in C282Y homozygotes (Rossi et al. 2001; Scotet et al. 2003; Burke et al. 2001). An increase in dietary heme iron intake also adversely affected the iron status in people with HFE defects (Greenwood et al. 2005; Pedersen and Milman 2009; van der et al. 2006). On the contrary, fruit juice excluding citrus fruits was demonstrated to be protective in hindering serum ferritin levels (Milward et al. 2008). The presence of dietary calcium and fiber protected against iron loading (Aranda et al. 2010). Thus, environmental or genetic factors likely influence the development of HH-related clinical symptoms (Olynyk et al. 2005).
We demonstrated that regulation of hepatic gene expression is strongly influenced by mouse strain and iron loading phase (age) in hfe−/− mouse groups. The effect of strain differences was likewise noted in studies of duodenal gene expression in four mouse strains (Dupic et al. 2002). In AKR hfe−/− mice, inappropriate down-regulation of hamp1 is consistent with the known role of hepcidin in regulating systemic iron metabolism. In C57 mice, hepcidin expression was not low, unless indexed to liver iron concentration. Inappropriately, low hepcidin relative to markers of iron status has also been reported in HH patients (Bridle et al. 2003; Piperno et al. 2007; van Dijk et al. 2008) and other hfe−/− mice models (Muckenthaler et al. 2003; Ahmad et al. 2002). Our results suggest that the milder phenotype of the C57 hfe−/− mice may be related to the relatively higher expression of hamp1 compared with AKR hfe−/− mice. Interestingly, however, the plateau phase observed in the AKR mice was not associated with up-regulation of liver hamp1 gene expression, and thus cannot be attributed to compensatory changes in signaling pathways regulating hepcidin. In the AKR mice, tfr1 a gene known to be down-regulated by cellular iron, demonstrated suppressed expression compared with wild-type mice only during the “loading” phase. This observation raises the possibility that fluxes in cellular iron status have greater influence on expression of this gene than does cellular iron stores. The C57 mice and 4-week-old AKR mice demonstrated reduced mRNA expression of Slc40a1, which encodes ferroportin, a key iron export protein (Abboud and Haile 2000; Knutson et al. 2003). The strong posttranslational regulation of ferroportin makes it difficult to determine the significance of these findings.
Microarray analysis also revealed that only a few genes were found in common between hfe−/− mice with different genetic backgrounds and between different age groups. Genetic variation, the degree of iron loading, and age appear to strongly influence the differences in target genes affected by defective hfe. However, we may have filtered out genes with small changes in expression during data analysis. There is limited information on the biological function of the few genes with greatest commonality across the study groups. Zdhhc14 and Gabra6 were commonly up-regulated in 4- and 8-week-old AKR hfe−/− mice. The biological function of ZDHHC14 is not known, but has been annotated as a probable palmitoyltransferase. The GABA-A receptor is highly expressed in neurons, where it plays a key role in GABA-mediated responses, but is still expressed in other tissues including the liver. Differential expression of Nfatc1 was commonly found in both 4-week-old AKR hfe−/− mice and 8-week-old C57 hfe−/− mice. NFATc1 is one of the several transcription factors that up-regulates proinflammatory cytokines and is involved in the development of fibrosis (Huang et al. 2002).
Although the lack of genes commonly affected in all hfe−/− mouse groups, biological functions related to cell survival, growth, and stress/damage response were commonly affected. These shared perturbations could contribute to the pathological conditions found in HH, such as hepatocellular carcinoma (Elmberg et al. 2003; Strohmeyer et al. 1988; Bradbear et al. 1985; Niederau et al. 1985). Our observation of up-regulation of cell proliferation genes in all hfe−/− mouse groups is in accordance with previous findings (Hann et al. 1990; Smith and Yeoh 1996; Pigeon et al. 1999). Increased cell growth was shown in an iron-treated human hepatoma cell line (Hann et al. 1990) and in animals grown on a carbonyl iron-supplemented diet (Smith and Yeoh 1996; Pigeon et al. 1999). This enhanced cell proliferation may play a role in the increased risk for hepatocellular carcinoma in patients with HH (Elmberg et al. 2003; Strohmeyer et al. 1988; Bradbear et al. 1985; Niederau et al. 1985). Up-regulation of genes in cell growth could be partly explained by cell damage caused by high hepatic iron content and the initiation of cell proliferation to compensate for the loss of damaged cells due to Hfe defects. Groups of genes in stress and damage response were altered in our study of hfe−/− mouse groups as compared to WT groups. These responses might be induced by high iron loading in hfe−/− mice, which can cause oxidative stress by reducing antioxidant levels and generating reactive oxygen species. Increased oxidative stress was reported in HH patients (Houglum et al. 1997) and in hfe−/− mice (Stevens et al. 2003).
Strain-specific differences in biological groups affected by hfe−/− could provide insight into the variable phenotypic manifestations of patients with HH. The iron plateau phase of AKR hfe−/− mice showed disturbed gene expression profiles in fibrosis, transport, oxidative phosphorylation, and β-amyloid production, whereas the iron plateau phase of C57 hfe−/−mice had differential expression of genes related to nutrient metabolism, cell adhesion, vesicle trafficking, RNA splicing, and calcium homeostasis. The effects of hepatic iron content on gene expression can be discerned by comparing different iron loading phases in hfe−/− mice. Unlike the iron plateau phase, the iron loading phase of AKR hfe−/− mice had dysregulated genes in drug metabolism, immune response, and extracellular matrix. Therefore, the differences in biological groups affected by differences in strains and/or iron loading status could be in part due to differences in hepatic iron content. Hepatic iron loading was greatest in 8-week-old C57BL/6J hfe−/− mice, followed by 4-week-old AKR/J hfe−/− mice, and was least in 8-week-old AKR/J hfe−/− mice. We suggest that the divergent phenotypes likely result from a complex interaction of the Hfe mutation with the different genetic backgrounds, consequent different rates of iron loading status, and the compensatory cellular response in the liver to the different levels of iron loading.
References
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912
Adams LA, Angulo P, Abraham SC, Torgerson H, Brandhagen D (2006a) The effect of the metabolic syndrome, hepatic steatosis and steatohepatitis on liver fibrosis in hereditary hemochromatosis. Liver Int 26:298–304
Adams PC, Passmore L, Chakrabarti S, Reboussin DM, Acton RT, Barton JC, McLaren GD, Eckfeldt JH, Dawkins FW, Gordeuk VR, Harris EL, Leiendecker-Foster C, Gossman E, Sholinsky P (2006b) Liver diseases in the hemochromatosis and iron overload screening study. Clin Gastroenterol Hepatol 4:918–923
Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, Sly WS, Fleming RE (2002) Decreased liver hepcidin expression in the hfe knockout mouse. Blood Cells Mol Dis 29:361–366
Aranda N, Viteri FE, Montserrat C, Arija V (2010) Effects of C282Y, H63D, and S65C HFE gene mutations, diet, and life-style factors on iron status in a general Mediterranean population from Tarragona, Spain. Ann Hematol 89:767–773
Asberg A, Hveem K, Thorstensen K, Ellekjter E, Kannelonning K, Fjosne U, Halvorsen TB, Smethurst HB, Sagen E, Bjerve KS (2001) Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol 36:1108–1115
Bacon BR, Powell LW, Adams PC, Kresina TF, Hoofnagle JH (1999) Molecular medicine and hemochromatosis: at the crossroads. Gastroenterology 116:193–207
Beutler E (2006) Hemochromatosis: genetics and pathophysiology. Annu Rev Med 57:331–347
Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T (2002) Penetrance of 845G->A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 359:211–218
Bradbear RA, Bain C, Siskind V, Schofield FD, Webb S, Axelsen EM, Halliday JW, Bassett ML, Powell LW (1985) Cohort study of internal malignancy in genetic hemochromatosis and other chronic nonalcoholic liver diseases. J Natl Cancer Inst 75:81–84
Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DHG, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA (2003) Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet 361:669
Bulaj ZJ, Griffen LM, Jorde LB, Edwards CQ, Kushner JP (1996) Clinical and biochemical abnormalities in people heterozygous for hemochromatosis. N Engl J Med 335:1799–1805
Bulaj ZJ, Ajioka RS, Phillips JD, LaSalle BA, Jorde LB, Griffen LM, Edwards CQ, Kushner JP (2000) Disease-related conditions in relatives of patients with hemochromatosis. N Engl J Med 343:1529–1535
Burke W, Imperatore G, Reyes M (2001) Iron deficiency and iron overload: effects of diet and genes. Proc Nutr Soc 60:73–80
Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P (2000) The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet 25:14–15
Dupic F, Fruchon S, Bensaid M, Loreal O, Brissot P, Borot N, Roth MP, Coppin H (2002) Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut 51:648–653
Elmberg M, Hultcrantz R, Ekbom A, Brandt L, Olsson S, Olsson R, Lindgren S, Loof L, Stal P, Wallerstedt S, Almer S, Sandberg-Gertzen H, Askling J (2003) Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 125:1733
Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13:399–408
Feder JN, Tsuchihashi Z, Irrinki A, Lee VK, Mapa FA, Morikang E, Prass CE, Starnes SM, Wolff RK, Parkkila S, Sly WS, Schatzman RC (1997) The hemochromatosis founder mutation in HLA-H disrupts beta 2-microglobulin interaction and cell surface expression. J Biol Chem 272:14025–14028
Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC (1998) The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. PNAS 95:1472–1477
Fleming RE, Sly WS (2002) Mechanisms of iron accumulation in hereditary hemochromatosis. Annu Rev Physiol 64:663–680
Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, Bacon BR, Roopenian DC, Sly WS (2001) Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA 98:2707–2711
Greenwood DC, Cade JE, Moreton JA, O’Hara B, Burley VJ, Randerson-Moor JA, Kukalizch K, Thompson D, Worwood M, Bishop DT (2005) HFE genotype modifies the influence of heme iron intake on iron status. Epidemiology 16:802–805
Gross CN, Irrinki A, Feder JN, Enns CA (1998) Co-trafficking of HFE, a nonclassical major histocompatibility complex class I protein, with the transferrin receptor implies a role in intracellular iron regulation. J Biol Chem 273:22068–22074
Hann HW, Stahlhut MW, Hann CL (1990) Effect of iron and desferoxamine on cell growth and in vitro ferritin synthesis in human hepatoma cell lines. Hepatology 11:566–569
Houglum K, Ramm GA, Crawford DH, Witztum JL, Powell LW, Chojkier M (1997) Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology 26:605–610
Huang C, Li J, Zhang Q, Huang X (2002) Role of bioavailable iron in coal dust-induced activation of activator protein-1 and nuclear factor of activated T cells: difference between pennsylvania and utah coal dusts. Am J Respir Cell Mol Biol 27:568–574
Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M (2003) Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood 102:4191–4197
Leboeuf RC, Tolson D, Heinecke JW (1995) Dissociation between tissue iron concentrations and transferrin saturation among inbred mouse strains. J Lab Clin Med 126:128–136
Lee PL, Beutler E, Rao SV, Barton JC (2004) Genetic abnormalities and juvenile hemochromatosis: mutations of the HJV gene encoding hemojuvelin. Blood 103:4669–4671
Loguinov A, Mian IS, Vulpe C (2004) Exploratory differential gene expression analysis in microarray experiments with no or limited replication. Genome Biol 5:R18
Milward EA, Baines SK, Knuiman MW, Bartholomew HC, Divitini ML, Ravine DG, Bruce DG, Olynyk JK (2008) Noncitrus fruits as novel dietary environmental modifiers of iron stores in people with or without HFE gene mutations. Mayo Clin Proc 83:543–549
Muckenthaler M, Roy CN, Custodio AO, Minana B, deGraaf J, Montross LK, Andrews NC, Hentze MW (2003) Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet 34:102–107
Mura C, Le Gac G, Scotet V, Raguenes O, Mercier A-Y, Ferec C (2001) Variation of iron loading expression in C282Y homozygous haemochromatosis probands and sib pairs. J Med Genet 38:632–636
Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G (1985) Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 313:1256–1262
Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW (1999) A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 341:718–724
Olynyk JK, St Pierre TG, Britton RS, Brunt EM, Bacon BR (2005) Duration of hepatic iron exposure increases the risk of significant fibrosis in hereditary hemochromatosis: a new role for magnetic resonance imaging. Am J Gastroenterol 100:837–841
Pedersen P, Milman N (2009) Extrinsic factors modifying expressivity of the HFE variant C282Y, H63D, S65C phenotypes in 1,294 Danish men. Ann Hematol 88:957–965
Pigeon C, Turlin B, Iancu TC, Leroyer P, Le Lan J, Deugnier Y, Brissot P, Loreal O (1999) Carbonyl-iron supplementation induces hepatocyte nuclear changes in BALB/CJ male mice. J Hepatol 30:926–934
Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E, Phung Y, Ganz T, Camaschella C (2007) Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood 110:4096–4100
Rasmussen ML, Folsom AR, Catellier DJ, Tsai MY, Garg U, Eckfeldt JH (2001) A prospective study of coronary heart disease and the hemochromatosis gene (HFE) C282Y mutation: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis 154:739–746
Rochette J, Le Gac G, Lassoued K, Ferec C, Robson KJ (2010) Factors influencing disease phenotype and penetrance in HFE haemochromatosis. Hum Genet 128:233–248
Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C (2003) Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 33:21–22
Rossi E, Bulsara MK, Olynyk JK, Cullen DJ, Summerville L, Powell LW (2001) Effect of hemochromatosis genotype and lifestyle factors on iron and red cell indices in a community population. Clin Chem 47:202–208
Roy CN, Penny DM, Feder JN, Enns CA (1999) The hereditary hemochromatosis protein, HFE, specifically regulates transferrin-mediated iron uptake in HeLa cells. J Biol Chem 274:9022–9028
Sanchez M, Villa M, Ingelmo M, Sanz C, Bruguera M, Ascaso C, Oliva R (2003) Population screening for hemochromatosis: a study in 5370 Spanish blood donors. J Hepatol 38:745–750
Scotet V, Merour MC, Mercier AY, Chanu B, Le Faou T, Raguenes O, Le Gac G, Mura C, Nousbaum JB, Ferec C (2003) Hereditary hemochromatosis: effect of excessive alcohol consumption on disease expression in patients homozygous for the C282Y mutation. Am J Epidemiol 158:129–134
Smith PG, Yeoh GC (1996) Chronic iron overload in rats induces oval cells in the liver. Am J Pathol 149:389–398
Stevens RG, Morris JE, Cordis GA, Anderson LE, Rosenberg DW, Sasser LB (2003) Oxidative damage in colon and mammary tissue of the HFE-knockout mouse. Free Radic Biol Med 34:1212–1216
Strohmeyer G, Niederau C, Stremmel W (1988) Survival and causes of death in hemochromatosis. Observations in 163 patients. Ann N Y Acad Sci 526:245–257
van der AD, Peeters PH, Grobbee DE, Roest M, Voorbij HA, van der Schouw YT (2006) HFE genotypes and dietary heme iron: no evidence of strong gene-nutrient interaction on serum ferritin concentrations in middle-aged women. Nutr Metab Cardiovasc Dis NMCD 16:60–68
van Dijk BA, Laarakkers CM, Klaver SM, Jacobs EM, van Tits LJ, Janssen MC, Swinkels DW (2008) Serum hepcidin levels are innately low in HFE-related haemochromatosis but differ between C282Y-homozygotes with elevated and normal ferritin levels. Br J Haematol 142:979–985
Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, Schatzman RC, Britton RS, Bacon BR, Sly WS (1997) Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta 2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7쟠ells. PNAS 94:12384–12389
Whitfield JB, Cullen LM, Jazwinska EC, Powell LW, Heath AC, Zhu G, Duffy DL, Martin NG (2000) Effects of HFE C282Y and H63D polymorphisms and polygenic background on iron stores in a large community sample of twins. Am J Hum Genet 66:1246–1258
Acknowledgments
This research was supported by an NIH Grant R01 DK095112.
Conflict of interest
No conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, SM., Loguinov, A., Fleming, R.E. et al. Effects of strain and age on hepatic gene expression profiles in murine models of HFE-associated hereditary hemochromatosis. Genes Nutr 10, 443 (2015). https://doi.org/10.1007/s12263-014-0443-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12263-014-0443-1