Abstract
Background
The primary aim of this study was to compare quality of life of postmenopausal breast cancer women receiving adjuvant hormone therapy with those not receiving. The secondary aims were to investigate impact of vitamin D status and type of hormone therapy (letrozole vs tamoxifen) on quality of life.
Settings and Design
Tertiary care hospital, prospective study (March 2016 to September 2017).
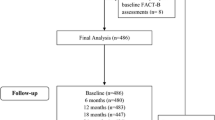
Patients and Methods
Study included postmenopausal non-metastatic breast cancer women. Patients were divided in two groups: group 1 (n=120) receiving adjuvant hormone therapy and group 2 (n=93) not receiving hormone therapy. Patients filled Functional Assessment of Cancer Therapy-Breast (FACT–B) QoL questionnaire at enrolment and 6 month later. The scores were compared between the groups.
Results
Overall basal FACT-G (p=0.004), FACT-B (p=0.003), and all other quality of life subscales scores barring social well-being were significantly better in group 2 {physical well-being (p=0.002), social well-being (p=0.037), emotional well-being (p=0.028), functional well-being (p=<0.001)}. Follow-up scores in all subscales within both group improved significantly (p=<0.001), but significant difference between two groups persisted only for FACT-G (p=0.04), physical well-being (p=0.02), and functional well-being (p=<0.001) subscale. Except for social well-being subscale, vitamin D sufficient patients had significantly better scores than deficient patients. There was no significant difference among patients receiving either letrozole or tamoxifen except for better functional well-being (p=0.005) score in those receiving previous.
Conclusions
Adjuvant hormonal therapy irrespective of type and vitamin D deficiency contribute to inferior quality of life in postmenopausal breast cancer women.
Similar content being viewed by others
References
National Cancer Registry Programme (2001) Consolidated report of the population based cancer registries 1990–1996. Indian Council of Medical Research, New Delhi
Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63:181–187
Foulkes WD, Reis-Filho JS, Narod SA (2010) Tumor size and survival in breast cancer: a reappraisal. Nat Rev Clin Oncol 7:348–353
Davies C, Godwin J, Gray R et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–845
Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE et al (2014) Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol 32:2255–2269
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR (2016) Extending aromatase – inhibitor adjuvant therapy to 10 years. N Engl J Med 375:209–219
ATAC Trialists’ Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of post-menopausal women with early-stage breast cancer: results of the ATAC (arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer 98:1802–1810
Van der Steeg AF, De Vries J, Roukema JA (2004) Quality of life and health status in breast carcinoma. Eur J Surg Oncol 30:1051–1057
Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107:167–180
Sert F, Ozsaran Z, Eser E, Alanyal SD, Haydaroglu A, Aras A (2013) Quality of life assessment in women with breast cancer: a prospective study including hormonal therapy. J Breast Cancer 16:220–228
Aparna P, Muthathal S, Nongkynrih B, Gupta SK (2018) Vitamin D deficiency in India. Fam Med Prim Care 7:324–330
Hollick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Callegari ET, Reavley N, Garland SM, Gorelik A, Wark JD (2015) Vitamin D status, bone mineral density and mental health in young Australian women: the safe-D study. J Public Health Res 4:594
Raczkiewicz A, Kisiel B, Kulig M, Tłustochowicz W (2015) Vitamin D status and its association with quality of life, physical activity, and disease activity in rheumatoid arthritis patients. J Clin Rheumatol 21:126–130
Hortobagyi GN et al. Breast. In Amin MB et al (edi.). AJCC cancer staging manual. Eight edition: p 589
Elizabeth H, Hammond H, Hayes DF, Dowsett M, Allered DC, Hagerty KL et al (2010) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol 28:2784–2795
NCCN Practice Guidelines in Oncology. v.1.2005. www.nccn.org/professional/physician-gls/PDF/breast.pdf.
WHO Scientific Group on the Prevention and Management of Osteoporosis (2003) Prevention and management of osteoporosis: report of a WHO scientific group. Geneva: World Health Organization. World Health Organ Tech Rep Ser 921:1–164
Kaul R, Sharma J, Minhas SS, Mardi K (2011) Hormone receptor status of breast cancer in the Himalayan Region of Northern India. Indian J Surg 73:9–12
Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A (2011) Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev 12:625–629
Grant WB (2015) 25-Hydroxyvitamin D and breast cancer, colorectal cancer and colorectal adenomas: case-control versus nested case control studies. Anticancer Res 35:1153–1160
Sofi NY, Jain M, Kapil U et al (2016) Nutritional risk factors and status of serum 25(OH)D levels in patients with breast cancer : a case control study in India. J Steroid Biochem Mol Biol 177:55–59
Bianchi ML, Orsini MR, Saraifoger S, Ortolani S, Radaelli BS (2005) Quality of life in post-menopausal osteoporosis. Health Qual Life Outcomes 3:78. https://doi.org/10.1186/1477-7525-3-78
Fallowfield LJ, Bliss JM, Porter LS, Price MH, Snowdon CF, Jones SE, Coombes RC, Hall E (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24:910–917
Pandey M, Thomas BC, Ramdas K, Eremenco S, Nair MK (2002) Quality of life in breast cancer patients: validation of a FACT-B Malayalam version. Qual Life Res 11:87–90
Hahn EA, Holzner B, Kemmler G, Sperner-Unterweger B, Hudgens SA, Cella D (2005) Cross-cultural evaluation of health status using item response theory: FACT-B comparisons between Austrian and U.S. patients with breast cancer. Eval Health Prof 28:233–259. https://doi.org/10.1177/0163278705275343
Acknowledgements
The authors acknowledge and thank “Functional Assessment of Chronic Illness Therapy (FACIT)” organization for the permission to use FACT-B Hindi questionnaire and also providing scoring material.
Availability of Data and Material
Data is property of the Institute and would be made available on specific request.
Code Availability
Not applicable
Author information
Authors and Affiliations
Contributions
☑ Contribution details (to be ticked marked as applicable)
Contributor 1Contributor 2Contributor 3Contributor 4Contributor 5Contributor 6Contributor 7
Concepts☑☑☑
Design☑☑☑
Definition of intellectual content☑☑☑☑☑☑☑
Literature search☑☑☑☑☑☑
Clinical studies☑☑☑☑☑☑☑
Experimental studies☑☑☑☑☑☑☑
Data acquisition☑
Data analysis☑☑☑☑☑☑☑
Statistical analysis☑☑☑☑
Manuscript preparation☑☑☑☑
Manuscript editing☑☑☑☑☑☑☑
Manuscript review☑☑☑☑☑☑☑
Guarantor☑☑
Corresponding author
Ethics declarations
Ethics Approval
The Institute Ethics Committee approved the study (2016-75-MCH-EXP).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The manuscript publication is approved by all authors and by the responsible authorities where the work was carried out.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A part of this study was presented as poster in 77th Annual National Conference of the Association of Surgeons of India (ASICON 2017) held at Jaipur from December 26 to 30, 2017.
Rights and permissions
About this article
Cite this article
Bichoo, R.A., Mishra, A., Lal, P. et al. Quality of Life (QoL) in Postmenopausal Breast Cancer Patients Receiving Adjuvant Hormonal Therapy. Indian J Surg 83 (Suppl 2), 461–467 (2021). https://doi.org/10.1007/s12262-021-02766-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-021-02766-6