Abstract
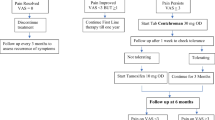
We conducted a randomized trial to evaluate effectiveness of Centchroman in control of mastalgia and compared it with Danazol. Research Question- Is proportion of pain relief achieved by Centchroman similar to or inferior to that achieved by Danazol? In a randomized controlled trial of Centchroman vs. Danazol in mastalgia, 81 patients with mastalgia were studied. Thirty-nine patients were randomized to Danazol arm and 42 in Centchroman arm. The treatment was given for 12 weeks, followed by observation for 12 weeks. The pain was measured by visual analogue scale (VAS) of 0–10. At 12 weeks 89.7% women achieved reduction in pain score to ≤3 in Centchroman group (pvalue 0.001). In Danazol group 69.44% women achieved reduction in pain score to ≤ 3 (p = 0.001). Three months after stopping therapy, Centchroman was more effective in pain score reduction at 24 weeks as compared to Danazol (p = 0.019). Centchroman is an effective, safe and inexpensive alternative to Danazol for treatment of mastalgia.



Similar content being viewed by others
References
Preece PE, Mansel RE, Bolton PM, Hughes LE, Baum M, Gravelle IH (1976) Clinical syndromes of mastalgia. Lancet 2:670–3
Potten CS, Watson RJ, Williams GT et al (1988) The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer 58:163–70
Hughes LE, Mansel RE, Webster DJT (2000) Problems of concept and nomenclature of benign breast disease. Benign disorders and diseases of the breast, 2nd edn. Saunders, London, p 15
Asch RH, Greenblatt RB (1977) The use of an impeded androgen Danazol in the management of benign breast disorders. Am J Obstet Gynecol 127:130
Mansel RE, Preece PE, Hughes LE (1978) A double blind trial of the prolactin inhibitor bromocriptine in painful benign breast disease. Brit J Surg 65:724–727
Fentiman IS, Caleffi M, Hamed H (1989) Dosage and duration of tamoxifen for mastalgia. A controlled trial. Br J Surg 76:901–904
Mansel RE, Goyal A, Preece P, Leinster S, Maddox PR, Gateley C, Kubista E, von Fournier D (2004) European randomized, multicenter study of goserline (Zoladex) in the management of mastalgia. Am J Obstet Gynecol 191(6):1942–9
Colak T, Ipek T, Kanik A, Ogetman Z, Aydin S (2003) Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg 196(4):525–30
Barros AC, Mottola J, Ruiz CA (1999) Reassurance in the treatment of mastalgia. Breast J 5:162
Hadi MS (2000) Sports Brassiere; Is it a solution for mastalgia? Breast J 6:407
Minton JP, Foeking MK, Webster DJT et al (1979) Response of fibrocystic disease to caffeine withdrawal and correlation with cystic nucleotides with breast disease. Am J Obstet Gynecol 135:157
Goyal A, Mansel RE (2005) A randomized multicenter study of gamolenic acid with and without, antioxidants, vitamins and minerals in the management of mastalgia. Breast J 11:41–47
Srivastava A, Mansel RE, Arvind N, Prasad K, Dhar A, Chabra A (2007) Evidence based management of Mastalgia: a meta-analysis of randomized trials. Breast 16:503–12
Hughes LE, Mansel RE, Webster DJT (2000) Problems of concept and nomenclature of benign breast disease. Benign Disorders and Diseases of the Breast, 2nd edn. Saunders, London, p 108
Mansel RE, Preece PE, Huges LE (1978) A double blind trial of prolactin inhibitor bromocriptine in painful benign breast disease. Br J Surg 65:724–727
Messinis LE, Lolis D (1988) Treatment of Premenstrual Mastalgia with Tamoxifen. Acta Obstet Gynecol Scand 67:307–309
Fentiman IS, Caleffi M, Brame K, Chaudary MA, Hayward JL (1986) Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet 1(8476):287–8
Kontostolis E, Stefanidis K, Navrozoglou I, Lolis D (1997) Comparison of Tamoxifen with Danazol for treatment of cyclical mastalgia. Gynecol Endocrinol 11:393–397
Singh MM, Centchroman (2001) A selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorder. Med Res Rev 21(4):302–347
Kamboj VP, Setty BS, Chandra H, Roy SK, Kar AB (1977) Biological profile of Centchroman—a new post-coital contraceptive. Indian J Exp Biol 15:1144–1150
Vaidya R, Joshi U, Meherji P, Rege N, Betrabet S, Joshi L, Sheth A, Devi PK (1977) Centchroman in healthy female volunteers. Indian J Exp Biol 15:1173–1176
Centchroman (1991) Multicentric trial with biweekly cum weekly dose. Central Drug Research Institute, Lucknow
Lal J (2010) Clinical pharmacokinetics and interaction of Centchroman- a mini review. Contraception. doi:10.1016/j.contraception.2009.11.007
Dhar A, Srivastava A (2007) Role of centchroman in regression of Mastalgia and Fibroadenoma. World J Surg 31:1178–84
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm1
(PDF 84 kb)
Appendix
Appendix
Sample Size Consideration
We put forth the assumption that, Centchroman therapy would achieve pain relief similar to or slightly less than that achieved by Danazol. Hence in this calculation one tailed hypothesis was considered. The clinically meaningful difference in the proportion of cases getting pain relief in between the two arms is represented by delta-(δ) which is the non-inferiority margin in calculating the sample size with the following null hypothesis (H0).
-
H0: ε ≤ δ, and alternative hypothesis HA: ε > δ , where ε is the difference in the proportion of success between two therapies. (ε = p1–p2 ; where p1 = pain response proportion with Centchroman therapy,
-
p2 = pain response proportion with Danazol therapy).
Allowing the 15% of difference between results of two therapies as the clinically meaningful difference, the value of δ has been chosen as 0.15.
-
Assuming: p1 = 50% .
-
The literature showed that 60% cases get benefit in mastalgia by Danazol therapy, hence value of p2 = 60% or 0.6.
-
The values of other factors were taken as follows:-Zœ = 1.645 for œ = 0.05 for one tailed hypothesis
-
Zβ = 0.84 for power = 80% for one or two tailed hypothesis
With all these values the formula used in calculation of sample size for one group was as follows: -
The value of N (i.e. the sample size for one group) was calculated to be 48. Expecting 10% of withdrawal of the volunteers from the groups, the total numbers of patients to participate were considered 110. The study presents the result on 81 subjects.
Method of Randomization
Block randomization with a block size of 4 was employed to generate a list of random numbers on web site www.randomization.com. The sealed numbered opaque brown envelopes were prepared with the label of case number e.g “ case 1” . The envelopes were opened after ensuring inclusion criteria and signing the consent form. The envelope contained a folded slip of paper stating the assigned treatment, i.e Centchroman or Danazol. The assigned treatment was given to the patient. No randomization violation or contamination occurred.
Rights and permissions
About this article
Cite this article
Tejwani, P.L., Srivastava, A., Nerkar, H. et al. Centchroman Regresses Mastalgia: A Randomized Comparison with Danazol. Indian J Surg 73, 199–205 (2011). https://doi.org/10.1007/s12262-010-0216-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-010-0216-z