Abstract
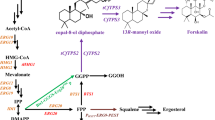
Tetraacetyl phytosphingosine (TAPS) is a precursor that has antibacterial and wound-healing properties and is used in the synthesis of ceramides. Wickerhamomyces ciferrii is a non-conventional yeast that has the ability to produce and secrete TAPS extracellularly. However, the genetic engineering tools available for this yeast species are limited. In this study, an auxotrophic strain Y94 of W. ciferrii was developed through random mutation and screening under 1.5 g/L 5-fluoroorotic acid. Additionally, an expression vector was constructed which harbors an uracil selection marker and CEN/ARS origin of replication. Using requisite mutations and expression vectors, we successfully constructed an engineered strain that overexpressed LCB1, LCB2, and SYR2, with the deletion of LCB4, resulting in the highest reported TAPS titer to date (20 g/L) through high cell density fermentation. This study demonstrates the potential of W. ciferrii for TAPS production and highlights the significance of an efficient genetic engineering toolbox in enhancing yields.
Similar content being viewed by others
References
Schorsch, C., T. Köhler, H. Andrea, and E. Boles (2012) High-level production of tetraacetyl phytosphingosine (TAPS) by combined genetic engineering of sphingoid base biosynthesis and L-serine availability in the non-conventional yeast Pichia ciferrii. Metab. Eng. 14: 172–184.
Börgel, D., M. van den Berg, T. Hüller, H. Andrea, G. Liebisch, E. Boles, C. Schorsch, R. van der Pol, A. Arink, I. Boogers, R. van der Hoeven, K. Korevaar, M. Farwick, T. Köhler, and S. Schaffer (2012) Metabolic engineering of the non-conventional yeast Pichia ciferrii for production of rare sphingoid bases. Metab. Eng. 14: 412–426.
Cowart, L. A. and Y. A. Hannun (2003) 12 Baker’s Yeast: a rising foundation for eukaryotic sphingolipid-mediated cell signaling. pp. 383–401. In: G. Daum (ed.). Lipid Metabolism and Membrane Biogenesis. Springer.
Cowart, L. A. and L. M. Obeid (2007) Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim. Biophys. Acta 1771: 421–431.
Dickson, R. C., C. Sumanasekera, and R. L. Lester (2006) Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog. Lipid Res. 45: 447–465.
Draelos, Z. D. (2008) The effect of ceramide-containing skin care products on eczema resolution duration. Cutis 81: 87–91.
Jang, E. J., Y. Shin, H. J. Park, D. Kim, C. Jung, J.-Y. Hong, S. Kim, and S. K. Lee (2017) Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J. Dermatol. Sci. 87: 19–28.
Jeon, J., M. Shin, J. W. Yoo, J. S. Oh, J. G. Bae, S. H. Jung, and Y. G. Kim (2007) Highly anti-selective dihydroxylation of 1,2-dialkyl substituted (Z)-allylic amines: stereoselective synthesis of a D-ribo-phytosphingosine derivative. Tetrahedron Lett. 48: 1105–1108.
Ceramide Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028. https://www.imarcgroup.com/ceramide-market
Choi, H. K., Y. H. Cho, E. O. Lee, J. W. Kim, and C. S. Park (2017) Phytosphingosine enhances moisture level in human skin barrier through stimulation of the filaggrin biosynthesis and degradation leading to NMF formation. Arch. Dermatol. Res. 309: 795–803.
Park, Y., K. S. Kim, M. Chung, J. H. Sung, and B. Kim (2016) Fabrication and characterization of dissolving microneedle arrays for improving skin permeability of cosmetic ingredients. J. Ind. Eng. Chem. 39: 121–126.
Školová, B., A. Kováčik, O. Tesař, L. Opálka, and K. Vávrová (2017) Phytosphingosine, sphingosine and dihydrosphingosine ceramides in model skin lipid membranes: permeability and biophysics. Biochim. Biophys. Acta 1859: 824–834.
Kwon, Y. B., C. D. Kim, B. J. Kim, M.-Y. Kim, C. S. Park, T.-J. Yoon, Y.-J. Seo, K.-B. Suhr, J.-K. Park, and J.-H. Lee (2007) Anti-angiogenic effect of tetraacetyl-phytosphingosine. Exp. Dermatol. 16: 311–317.
Barenholz, Y. and S. Gatt (1969) Acetylation of sphingosine bases and long-chain amines by cell-free preparations of Hansenula ciferri. Biochem. Biophys. Res. Commun. 35: 676–680.
Wickerham, L. J. and F. H. Stodola (1960) Formation of extracellular sphingolipides by microorganisms. I. Tetraacetylphyto-sphingosine from Hansenula ciferri. J. Bacteriol. 80: 484–491.
Schneider, J., H. Andrea, J. Blom, S. Jaenicke, C. Rückert, C. Schorsch, R. Szczepanowski, M. Farwick, A. Goesmann, A. Pühler, S. Schaffer, A. Tauch, T. Köhler, and K. Brinkrolf (2012) Draft genome sequence of Wickerhamomyces ciferrii NRRL Y-1031 F-60-10. Eukaryot. Cell 11: 1582–1583.
Choi, J. Y., H. J. Hwang, W. Y. Cho, J.-I. Choi, and P. C. Lee (2021) Differences in the fatty acid profile, morphology, and tetraacetylphytosphingosine-forming capability between wild-type and mutant Wickerhamomyces ciferrii. Front. Bioeng. Biotechnol. 9: 662979.
Ter Veld, F., D. Wolff, C. Schorsch, T. Köhler, E. Boles, and A. Poetsch (2013) Production of tetraacetyl phytosphingosine (TAPS) in Wickerhamomyces ciferrii is catalyzed by acetyltransferases Sli1p and Atf2p. Appl. Microbiol. Biotechnol. 97: 8537–8546.
Eun, S. W. and P. C. Lee (2023) Investigation of the effects of culture conditions on cell growth and tetraacetylphytosphingosine production by mutant Wickerhamomyces ciferrii. Process Biochem. 130: 203–210.
Park, S.-B., Q.-G. Tran, A. J. Ryu, J.-H. Yun, K. K. Kwon, Y. J. Lee, and H.-S. Kim (2022) Fluorescence-activated cell sorting-mediated directed evolution of Wickerhamomyces ciferrii for enhanced production of tetraacetyl phytosphingosine. Korean J. Chem. Eng. 39: 1004–1010.
Han, C., M. Jang, M. J. Kim, M.-H. Han, K.-R. Lee, J.-S. Hahn, and J. Ahn (2021) Engineering Yarrowia lipolytica for de novo production of tetraacetyl phytosphingosine. J. Appl. Microbiol. 130: 1981–1992.
Huang, L., J. Xu, T. Li, L. Wang, T. Deng, and X. Yu (2014) Effects of additional Mg2+ on the growth, lipid production, and fatty acid composition of Monoraphidium sp. FXY-10 under different culture conditions. Ann. Microbiol. 64: 1247–1256.
Lee, S.-M. and J.-H. Lee (2012) Ethanol fermentation for main sugar components of brown-algae using various yeasts. J. Ind. Eng. Chem. 18: 16–18.
Ko, J. K., Y. Um, H. M. Woo, K. H. Kim, and S.-M. Lee (2016) Ethanol production from lignocellulosic hydrolysates using engineered Saccharomyces cerevisiae harboring xylose isomerase-based pathway. Bioresour. Technol. 209: 290–296.
Hoang Nguyen Tran, P., J. K. Ko, G. Gong, Y. Um, and S.-M. Lee (2020) Improved simultaneous co-fermentation of glucose and xylose by Saccharomyces cerevisiae for efficient lignocellulosic biorefinery. Biotechnol. Biofuels 13: 12.
Kwak, S. and Y.-S. Jin (2017) Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microb. Cell Fact. 16: 82.
Bae, J.-H., J.-H. Sohn, C.-S. Park, J.-S. Rhee, and E.-S. Choi (2003) Integrative transformation system for the metabolic engineering of the sphingoid base-producing yeast Pichia ciferrii. Appl. Environ. Microbiol. 69: 812–819.
Marx, C. J. and M. E. Lidstrom (2002) Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33: 1062–1067.
Schorsch, C., T. Köhler, and E. Boles (2009) Knockout of the DNA ligase IV homolog gene in the sphingoid base producing yeast Pichia ciferrii significantly increases gene targeting efficiency. Curr. Genet. 55: 381–389.
Hastings, P. J., C. McGill, B. Shafer, and J. N. Strathern (1993) Ends-in vs. ends-out recombination in yeast. Genetics 135: 973–980.
García-Ríos, E., M. Lairón-Peris, S. Muñiz-Calvo, J. M. Heras, A. Ortiz-Julien, P. Poirot, N. Rozès, A. Querol, and J. M. Guillamón (2021) Thermo-adaptive evolution to generate improved Saccharomyces cerevisiae strains for cocoa pulp fermentations. Int. J. Food Microbiol. 342: 109077.
Luo, Z., K. Yu, S. Xie, M. Monti, D. Schindler, Y. Fang, S. Zhao, Z. Liang, S. Jiang, M. Luan, C. Xiao, Y. Cai, and J. Dai (2021) Compacting a synthetic yeast chromosome arm. Genome Biol. 22: 5.
Hashimoto, S., M. Ogura, K. Aritomi, H. Hoshida, Y. Nishizawa, and R. Akada (2005) Isolation of auxotrophic mutants of diploid industrial yeast strains after UV mutagenesis. Appl. Environ. Microbiol. 71: 312–319.
Laughery, M. F., T. Hunter, A. Brown, J. Hoopes, T. Ostbye, T. Shumaker, and J. J. Wyrick (2015) New vectors for simple and streamlined CRISPR-Cas9 genome editing in Saccharomyces cerevisiae. Yeast 32: 711–720.
Liu, M., X. Zhang, and T. Tan (2016) The effect of amino acids on lipid production and nutrient removal by Rhodotorula glutinis cultivation in starch wastewater. Bioresour. Technol. 218: 712–717.
Cai, Y., H. Chen, X. Tang, J. Zhao, H. Zhang, Y. Q. Chen, and W. Chen (2022) The relationship between amino acid and lipid metabolism in oleaginous eukaryotic microorganism. Appl. Microbiol. Biotechnol. 106: 3405–3417.
Sarma, S. J., R. K. Das, S. K. Brar, Y. L. Bihan, G. Buelna, M. Verma, and C. R. Soccol (2014) Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy 78: 16–22.
Verduyn, C., E. Postma, W. A. Scheffers, and J. P. V Dijken (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501–517.
Acknowledgements
This research was supported by Korea University funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yoo, S.W., Kim, M., Park, BS. et al. Wickerhamomyces ciferrii Auxotroph and Expression Vector for Improved Production of Tetraacetyl Phytosphingosine. Biotechnol Bioproc E 28, 804–812 (2023). https://doi.org/10.1007/s12257-023-0128-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-023-0128-y