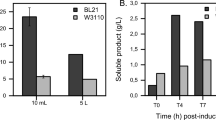
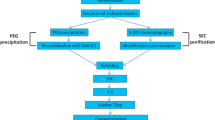
Abstract
The use of laboratory procedures is often inefficient for materialisation of recombinant therapeutic proteins in Escherichia coli (E. coli) for pre-clinical evaluation. Approaches such as scaling out shake flask cultivation can be laborious, inefficient and expensive. These inefficiencies can be compounded if the protein requires post-translational modification such as multimerisation. We previously used laboratory methods to produce the < 60 kDa, recombinant biotherapeutic, RB1. We were aware, a priori, that dimerisation of RB1 could double the molecular weight of the protein and increase its systemic retention in the human body by avoiding renal filtration. Here we modified RB1 by substituting a native residue for an unpaired cysteine, generating eRB1, in order to favour its dimerisation. Laboratory methods failed to achieve > 20% disulphide-bridged homodimerisation or monomer of sufficient purity to enable chemi-dimerisation. As such we established a set of high performance, bench-scale, unit operations for cultivation of E. coli cells expressing eRB1, the isolation of eRB1 inclusion bodies, refolding and disulphide-based dimerisation of ≥ 40% of total eRB1 and finally successful chemi-dimerisation of remaining monomeric eRB1. The establishment of scalable procedures can now enable future investigations of eRB1 and other < 60 kDa biologics for which significant bench-scale production is required for pre-clinical evaluation.
Similar content being viewed by others
References
Vazquez, E., J. Corchero, and A. Villaverde (2011) Post-production protein stability: Trouble beyond the cell factory. Microb. Cell Factories 10: 60–66.
Hellebust, H., M. Murby, L. Abrahmsén, M. Uhlén, and S. O. Enfors (1989) Different approaches to stabilize a recombinant fusion protein. Nat. Biotechnol. 7: 165–168.
Palmer, I. and P. T. Wingfield (2004) Preparation and extraction of insoluble (Inclusion-Body) proteins from Escherichia coli. In: J. E. Coligan, B. M. Dunn, D. W. Speicher, and P. T. Wingfield (eds.). Current Protocols in Protein Science. John Wiley & Sons Inc., Hoboken, NJ, USA.
Trusheim, M. R. and E. R. Berndt (2015) The clinical benefits, ethics, and economics of stratified medicine and companion diagnostics. Drug Discov. Today 20: 1439–1450.
Junttila, M. R. and F. J. de Sauvage (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501: 346–354.
Trivedi, M. V., J. S. Laurence, and T. J. Siahaan (2009) The role of thiols and disulfides on protein stability. Curr. Protein Pept. Sci. 10: 614–625.
Auclair, J. R., K. J. Boggio, G. A. Petsko, D. Ringe, and J. N. Agar (2010) Strategies for stabilizing superoxide dismutase (SOD1), the protein destabilized in the most common form of familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. 107: 21394–21399.
Prinz, W. A., F. Aslund, A. Holmgren, and J. Beckwith (1997) The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 272: 15661–15667.
Hermanson, G. T. (2008) Bioconjugate techniques. 2nd ed., pp. 1202, Academic Press, San Diego, USA.
Moore, J. E. and W. H. Ward (1956) Cross-linking of bovine plasma albumin and wool keratin. J. Am. Chem. Soc. 78: 2414–2418.
Yoshitake, S., M. Imagawa, E. Ishikawa, Y. Niitsu, I. Urushizaki, and M. Nishiura (1982) Mild and efficient conjugation of rabbit Fab’ and horseradish peroxidase using a maleimide compound and its use for enzyme immunoassay. J. Biochem. 92: 1413–1424.
Baldwin, A. D. and K. L. Kiick (2011) Tunable degradation of maleimide–thiol adducts in reducing environments. Bioconjug. Chem. 22: 1946–1953.
Fontaine, S. D., R. Reid, L. Robinson, G. W. Ashley, and D. V. Santi (2015) Long-term stabilization of maleimide–thiol conjugates. Bioconjug. Chem. 26: 145–152.
Ho, R. J. Y. and M. Gibaldi (2013) Biotechnology and biopharmaceuticals: Transforming proteins and genes into drugs. 2nd ed., pp.698, Wiley-Blackwell, Hoboken, NJ, USA.
Green, M. R. and J. Sambrook (2012) Molecular cloning: A laboratory manual. 4th ed., p. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA.
Matos, C. F. R. O., S. D. Branston, A. Albiniak, A. Dhanoya, R. B. Freedman and E. Keshavarz-Moore (2012) High-yield export of a native heterologous protein to the periplasm by the tat translocation pathway in Escherichia coli. Biotechnol. Bioeng. 109: 2533–2542.
Mannall, G. (2007) Characterisation of the effect of process factors upon protein refolding yield. Ph.D. Thesis. University College London, London, UK.
Nesbeth, D. N., M. A. Perez-Pardo, S. Ali, J. Ward, and E. Keshavarz-Moore (2012) Growth and productivity impacts of periplasmic nuclease expression in an Escherichia coli Fab’ fragment production strain. Biotechnol. Bioeng. 109: 517–527.
Guiliano, B., H. Fussell, I. Lenart, E. Tsao, D. N. Nesbeth, A. J. Fletcher, E. C. Campbell, N. Yousaf, S. Williams, A. Cameron, G. J. Towers, P. Kellam, D. N. Hebert, K. Gould, S. J. Powis, and A. N. Antoniou (2014) EDEM1 targets misfolded HLA-B27 dimers for endoplasmic reticulum associated degradation. Arthritis Rheumatol. 66: 2976–2988.
Zelikin, A. N., J. F. Quinn, and F. Caruso (2006) Disulfide crosslinked polymer capsules: En route to biodeconstructible systems. Biomacromol. 7: 27–30.
Ovsejevi, K., C. Manta, and F. Batista-Viera (2013) Reversible covalent immobilization of enzymes via disulfide bonds. Methods Mol. Biol. 1051: 89–116.
Wilbourn, B., D. N. Nesbeth, L. J. Wainwright, and M. C. Field (1998) Proteasome and thiol involvement in quality control of glycosylphosphatidylinositol anchor addition. Biochem. J. 332: 111–118.
Bessette, P. H. (1999) Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. 96: 13703–13708.
Thomas, J. G. and F. Baneyx (1996) Protein folding in the cytoplasm of Escherichia coli: requirements for the DnaK-DnaJGrpE and GroEL-GroES molecular chaperone machines. Mol. Microbiol. 21: 1185–1196.
Levy, R., R. Weiss, G. Chen, B. L. Iverson, and G. Georgiou (2001). Production of correctly folded Fab antibody fragment in the cytoplasm of Escherichia coli trxB gor mutants via the coexpression of molecular chaperones. Protein Expr. Purif. 23: 338–347.
Burgess, R. R. (2009). Refolding solubilized inclusion body proteins. Meth. Enzymol. 463: 259–282.
Tous, G. I., Z. Wei, J. Feng, S. Bilbulian, S. Bowen, and J. Smith (2005) Characterization of a novel modification to monoclonal antibodies: Thioether cross-link of heavy and light chains. Anal. Chem. 77: 2675–2682.
Smith, M. E. B., F. F. Schumacher, C. P. Ryan, L. M. Tedaldi, D. Papaioannou, and G. Waksman (2010) Protein modification, bioconjugation, and disulfide bridging using bromomaleimides. J. Am. Chem. Soc. 132: 19601965.
Kitagishi, H., H. Kawasaki, and K. Kano (2015) Bioconjugation of serum albumin to a maleimide-appended porphyrin/Cyclodextrin supramolecular complex as an artificial oxygen carrier in the bloodstream. Chem -Asian J. 10: 1768–1775.
Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl (1987) Current Protocols in Molecular Biology. 3rd ed. John Wiley and Sons Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schofield, D.M., Nesbeth, D.N. A high performance bench scale process for isolation from inclusion bodies, refolding and dimerisation of a thiol-engineered recombinant therapeutic protein. Biotechnol Bioproc E 22, 423–430 (2017). https://doi.org/10.1007/s12257-016-0385-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0385-0