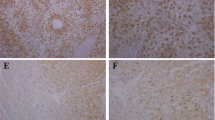
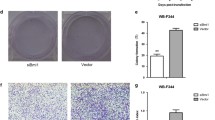
Abstract
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, and occurs in people with chronic liver diseases. Current treatment methods include surgery, transplant, and chemotherapy. Our study demonstrates runt-related transcription factor 1 (RUNX1) as a novel molecule in the initiation and development of HCC, and the role of its interaction with vascular endothelial growth factor A (VEGFA) in HCC. We showed the suppressive role of RUNX1 in the proliferation and migration of hepatocytes. In addition, the repressor RUNX1 functioned as a transcription factor on the promoter of VEGFA to inhibit the expression of VEGFA. Study in the HCC cells demonstrated that the suppression of HCC proliferation and migration was masked in the presence of overexpressed VEGFA. Introduction of RUNX1 into HCC mice model significantly limited the tumor growth. In summary, our study demonstrated that RUNX1 functions as a repressor in the HCC and this suppressive function was dependent on its effect on VEGFA.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, Pavarino EC, da Silva RF, da Silva RC, Goloni-Bertollo EM (2017) Hepatocellular Carcinoma: a Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac J Cancer Prev 18(4):863–872. https://doi.org/10.22034/APJCP.2017.18.4.863
Gomes MA, Priolli DG, Tralhão JG, Botelho MF (2013) Hepatocellular carcinoma: epidemiology, biology, diagnosis, and therapies. Rev Assoc Med Bras 59(5):514–524. https://doi.org/10.1016/j.ramb.2013.03.005
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379(9822):1245–1255. https://doi.org/10.1016/S0140-6736(11)61347-0
Forner A, Gilabert M, Bruix J, Raoul JL (2014) Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 11(9):525–535. https://doi.org/10.1038/nrclinonc.2014.122
Sood R, Kamikubo Y, Liu P (2017) Role of RUNX1 in hematological malignancies. Blood 129(15):2070–2082. https://doi.org/10.1182/blood-2016-10-687830
Ito Y, Bae SC, Chuang LS (2015) The RUNX family: developmental regulators in cancer. Nat Rev Cancer 15(2):81–95. https://doi.org/10.1038/nrc3877
Wu J, Huang G, Ratner N (2015) Runx1: a new driver in neurofibromagenesis. Oncoscience 2(11):904–905. https://doi.org/10.18632/oncoscience.266
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Proteomics. Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
Goyama S, Huang G, Kurokawa M, Mulloy JC (2015) Posttranslational modifications of RUNX1 as potential anticancer targets. Oncogene 34(27):3483–3492. https://doi.org/10.1038/onc.2014.305
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10. https://doi.org/10.1159/000088478
Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, Helfrich I (2012) Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med 209(11):2001–2016. https://doi.org/10.1084/jem.20111497
Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13(12):871–882. https://doi.org/10.1038/nrc3627
Weis SM, Cheresh DA (2005) Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437(7058):497–504. https://doi.org/10.1038/nature03987
Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153(1):13–19. https://doi.org/10.1093/jb/mvs136
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M (2015) VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212(2):139–148. https://doi.org/10.1084/jem.20140559
Horwitz E, Stein I, Andreozzi M, Nemeth J, Shoham A, Pappo O, Schweitzer N, Tornillo L, Kanarek N, Quagliata L, Zreik F, Porat RM, Finkelstein R, Reuter H, Koschny R, Ganten T, Mogler C, Shibolet O, Hess J, Breuhahn K, Grunewald M, Schirmacher P, Vogel A, Terracciano L, Angel P, Ben-Neriah Y, Pikarsky E (2014) Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov 4(6):730–743. https://doi.org/10.1158/2159-8290.CD-13-0782
Gascon E, Gaillard S, Malapert P, Liu Y, Rodat-Despoix L, Samokhvalov IM, Delmas P, Helmbacher F, Maina F, Moqrich A (2010) Hepatocyte growth factor-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons. J Neurosci 30(37):12414–12423. https://doi.org/10.1523/JNEUROSCI.3135-10.2010
Ter Elst A, Ma B, Scherpen FJ, de Jonge HJ, Douwes J, Wierenga AT, Schuringa JJ, Kamps WA, de Bont ES (2011) Repression of vascular endothelial growth factor expression by the runt-related transcription factor 1 in acute myeloid leukemia. Cancer Res 71(7):2761–2771. https://doi.org/10.1158/0008-5472.CAN-10-0402
Bowers SR, Calero-Nieto FJ, Valeaux S, Fernandez-Fuentes N, Cockerill PN (2010) Runx1 binds as a dimeric complex to overlapping Runx1 sites within a palindromic element in the human GM-CSF enhancer. Nucleic Acids Res 38(18):6124–6134. https://doi.org/10.1093/nar/gkq356
Klaunig JE, Pereira MA, Ruch RJ, Weghorst CM (1988) Dose-response relationship of diethylnitrosamine-initiated tumors in neonatal balb/c mice: effect of phenobarbital promotion. Toxicol Pathol 16(3):381–385. https://doi.org/10.1177/019262338801600310
Wang L, Brugge JS, Janes KA (2011) Intersection of FOXO- and RUNX1-mediated gene expression programs in single breast epithelial cells during morphogenesis and tumor progression. Proc Natl Acad Sci U S A 108(40):E803–E812. https://doi.org/10.1073/pnas.1103423108
Mercado-Matos J, Matthew-Onabanjo AN, Shaw LM (2017) RUNX1 and breast cancer. Oncotarget 8(23):36934–36935. https://doi.org/10.18632/oncotarget.17249
Barnum KJ, O'Connell MJ (2014) Cell cycle regulation by checkpoints. Methods Mol Biol 1170:29–40. https://doi.org/10.1007/978-1-4939-0888-2_2
Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ (2014) Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157(5):1146–1159. https://doi.org/10.1016/j.cell.2014.03.045
Battaglia RA, Delic S, Herrmann H, Snider NT (2018) Vimentin on the move: new developments in cell migration. F1000Res 7. https://doi.org/10.12688/f1000research.15967.1
Kidd ME, Shumaker DK, Ridge KM (2014) The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50(1):1–6. https://doi.org/10.1165/rcmb.2013-0314TR
Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B (2005) Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res 65(1):130–136
Srivastava VK, Gara RK, Rastogi N, Mishra DP, Ahmed MK, Gupta S, Goel MM, Bhatt ML (2014) Serum vascular endothelial growth factor-A (VEGF-A) as a biomarker in squamous cell carcinoma of head and neck patients undergoing chemoradiotherapy. Asian Pac J Cancer Prev 15(7):3261–3265
Author information
Authors and Affiliations
Contributions
CL and JH conceived and designed the experiments, DWX and BX analyzed and interpreted the results of the experiments, JL and BLL performed the experiments.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not Applicable.
Patient Consent for Publication
Not Applicable.
Competing Interests
The authors state that there are no conflicts of interest to disclose.
Informed Consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Xu, D., Xue, B. et al. Upregulation of RUNX1 Suppresses Proliferation and Migration through Repressing VEGFA Expression in Hepatocellular Carcinoma. Pathol. Oncol. Res. 26, 1301–1311 (2020). https://doi.org/10.1007/s12253-019-00694-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00694-1